▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk for the UK and hpra.ie/homepage/about-us/report-an-issue for Republic of Ireland. Adverse events should also be reported to UCB Pharma Ltd at ucbcares.uk@ucb.com or 0800 2793177 for the UK and UCB (Pharma) Ireland Ltd at ucbcares.ie@ucb.com or 1800 930075 for Republic of Ireland.
BIMZELX ACHIEVED A CONSISTENT RESPONSE ACROSS MANIFESTATIONS IN PsA WHICH WAS SUSTAINED OVER TIME1–9
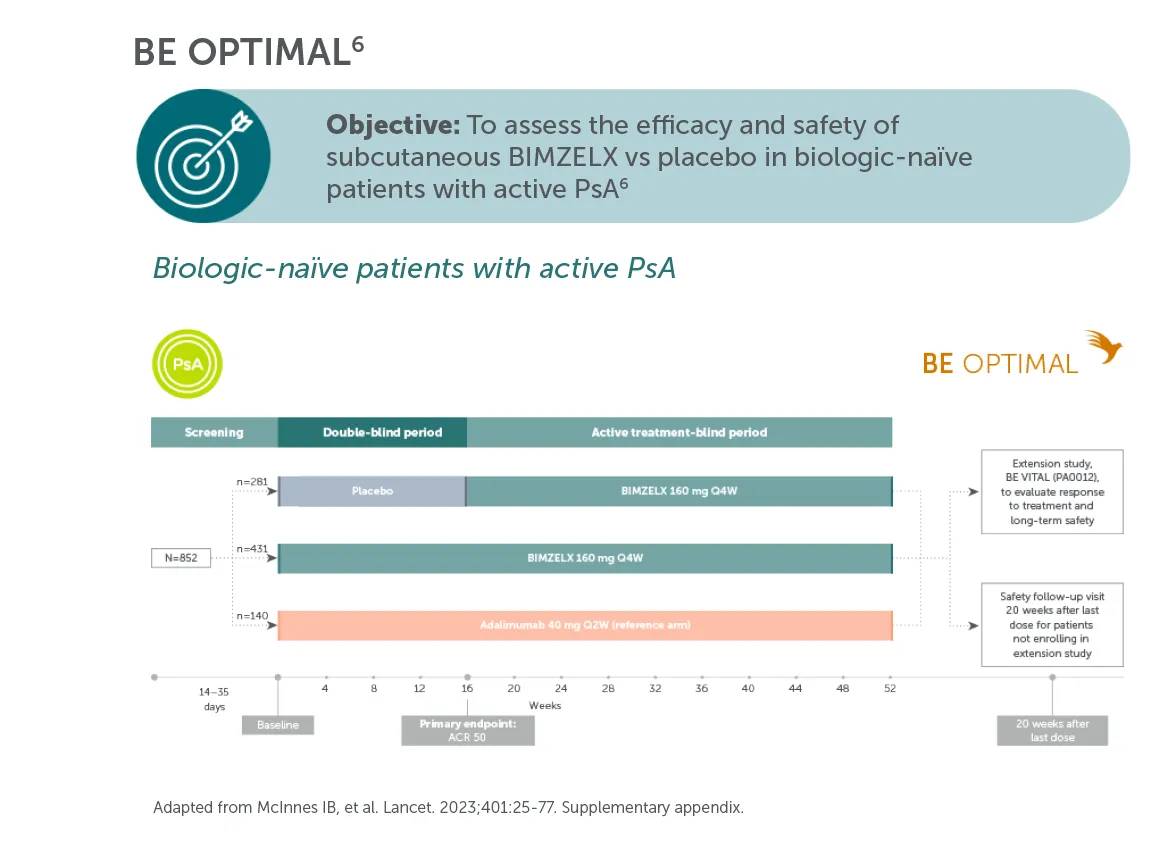
BIMZELX® (bimekizumab) is indicated for the treatment of: active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response or who have been intolerant to one or more DMARDs; active nr-axSpA, in adults with objective signs of inflammation as indicated by elevated CRP and/or MRI, who have responded inadequately or are intolerant to NSAIDs; and active AS, in adults who have responded inadequately or are intolerant to conventional therapy.4
CHALLENGE LIMITATIONS IN PsA WITH BIMZELX
EFFICACY
Explore more in ‘Symptom improvement’ section below |

Explore more in ‘Reductions in disease activity’ section below |

Explore more in ‘Inhibition of disease progression’ section below |
Explore more in ‘Resolution of key manifestations’ section below |

Explore more in ‘Resolution of peripheral arthritis’ section below |
Explore more in ‘Patient-reported outcomes’ section below |
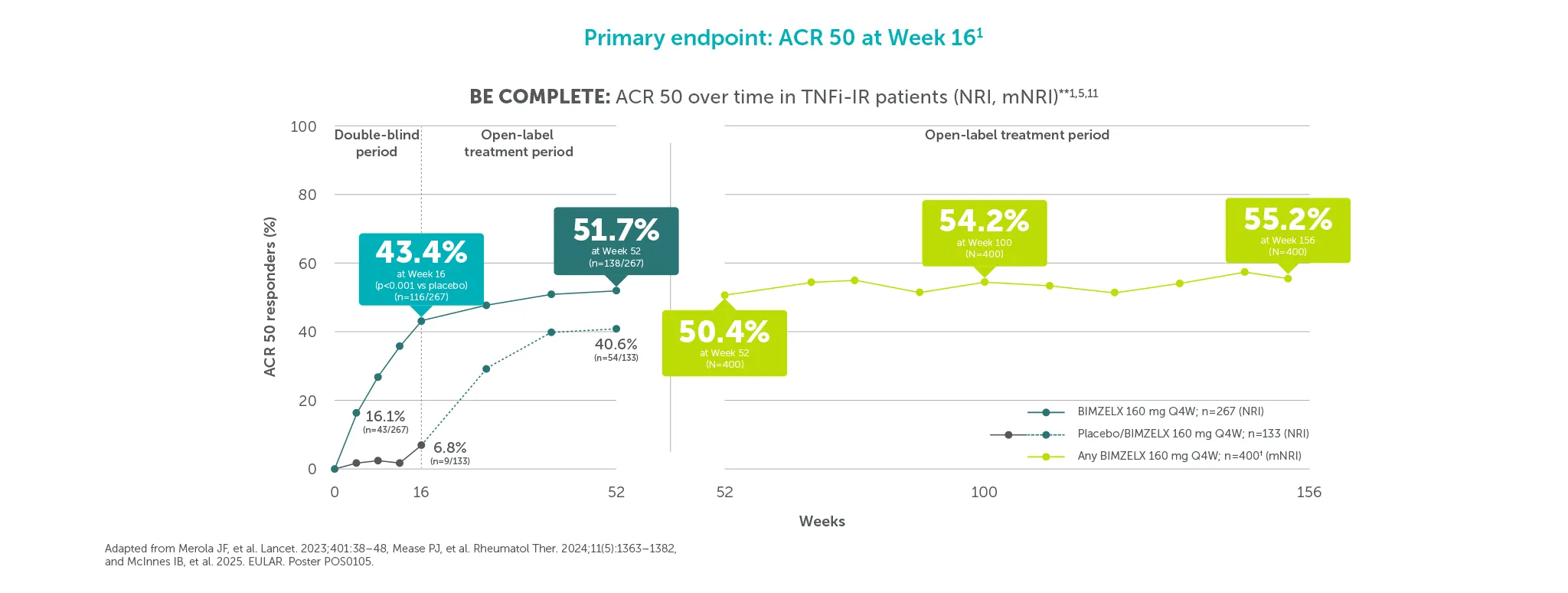
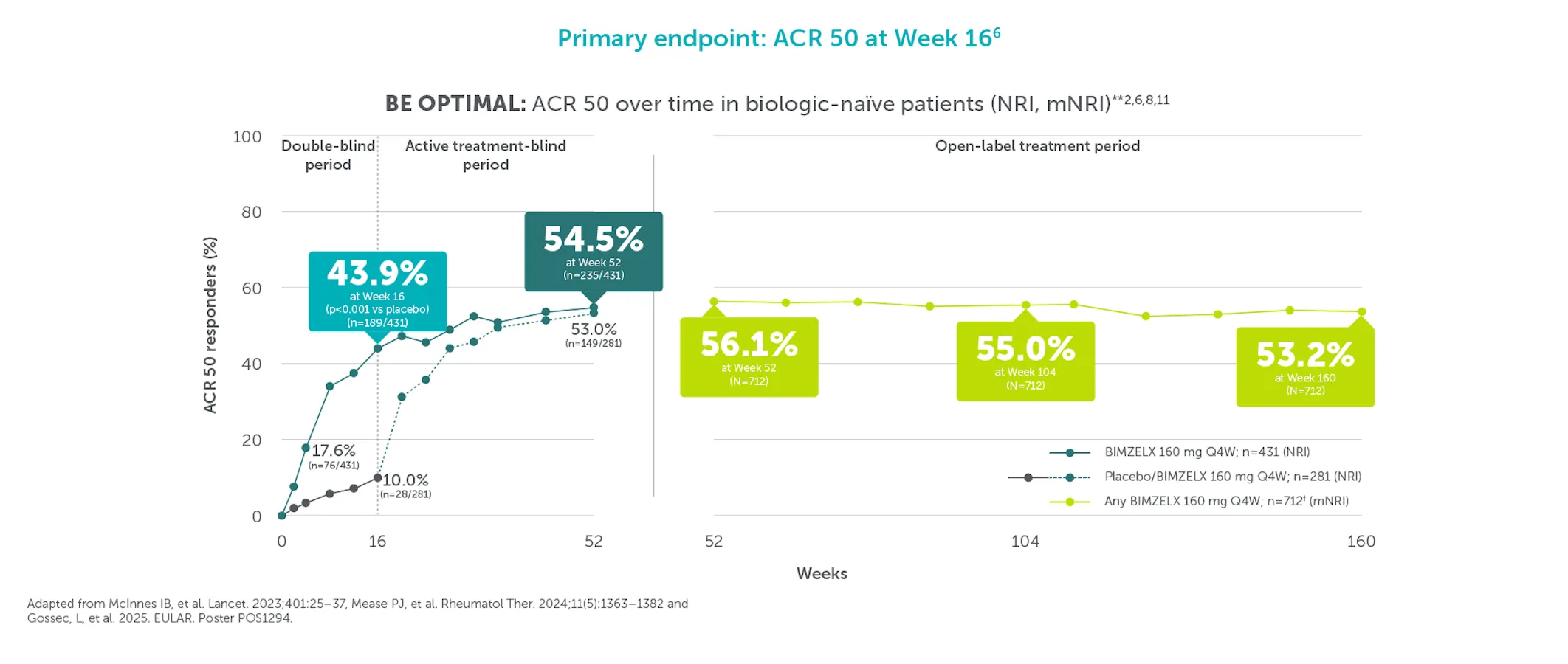
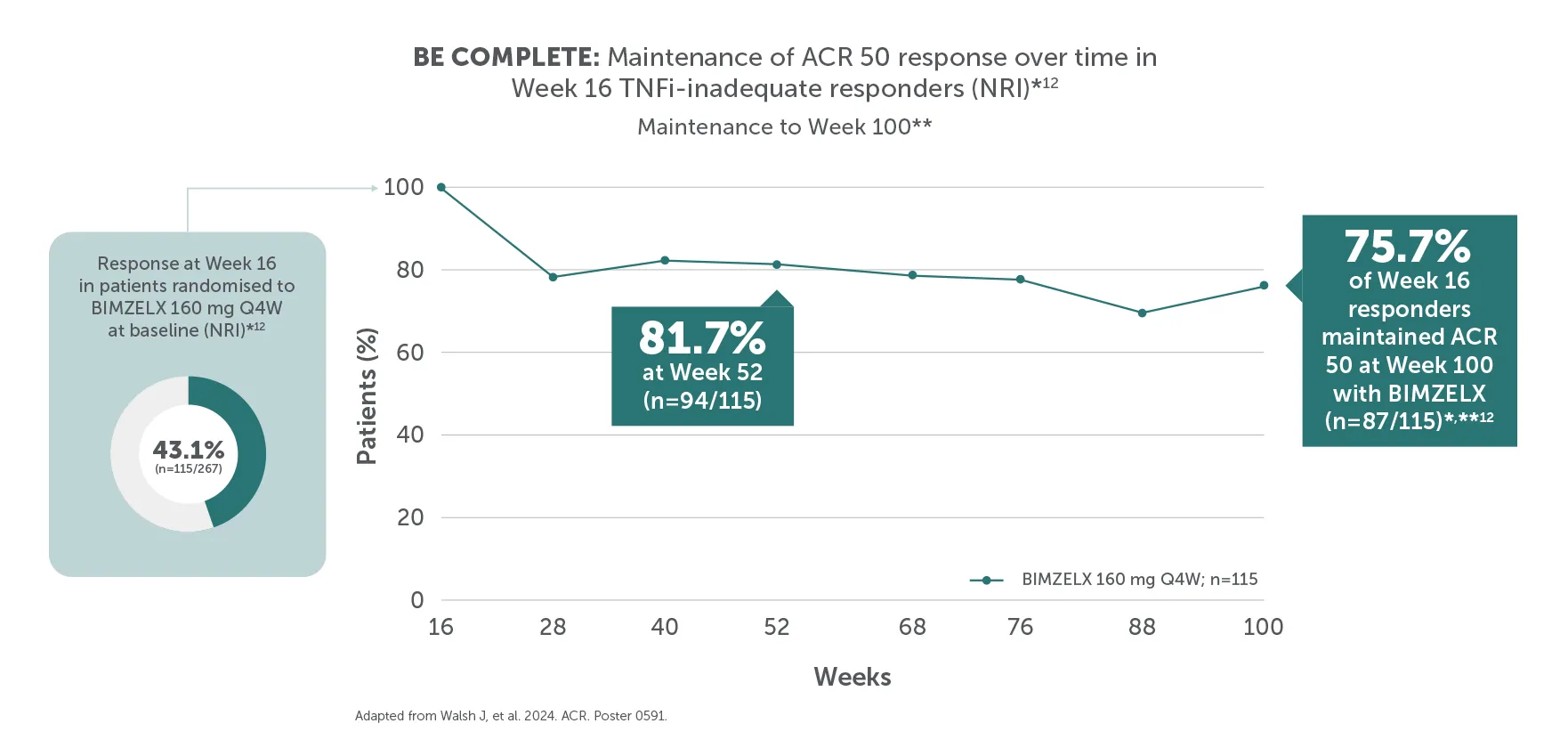
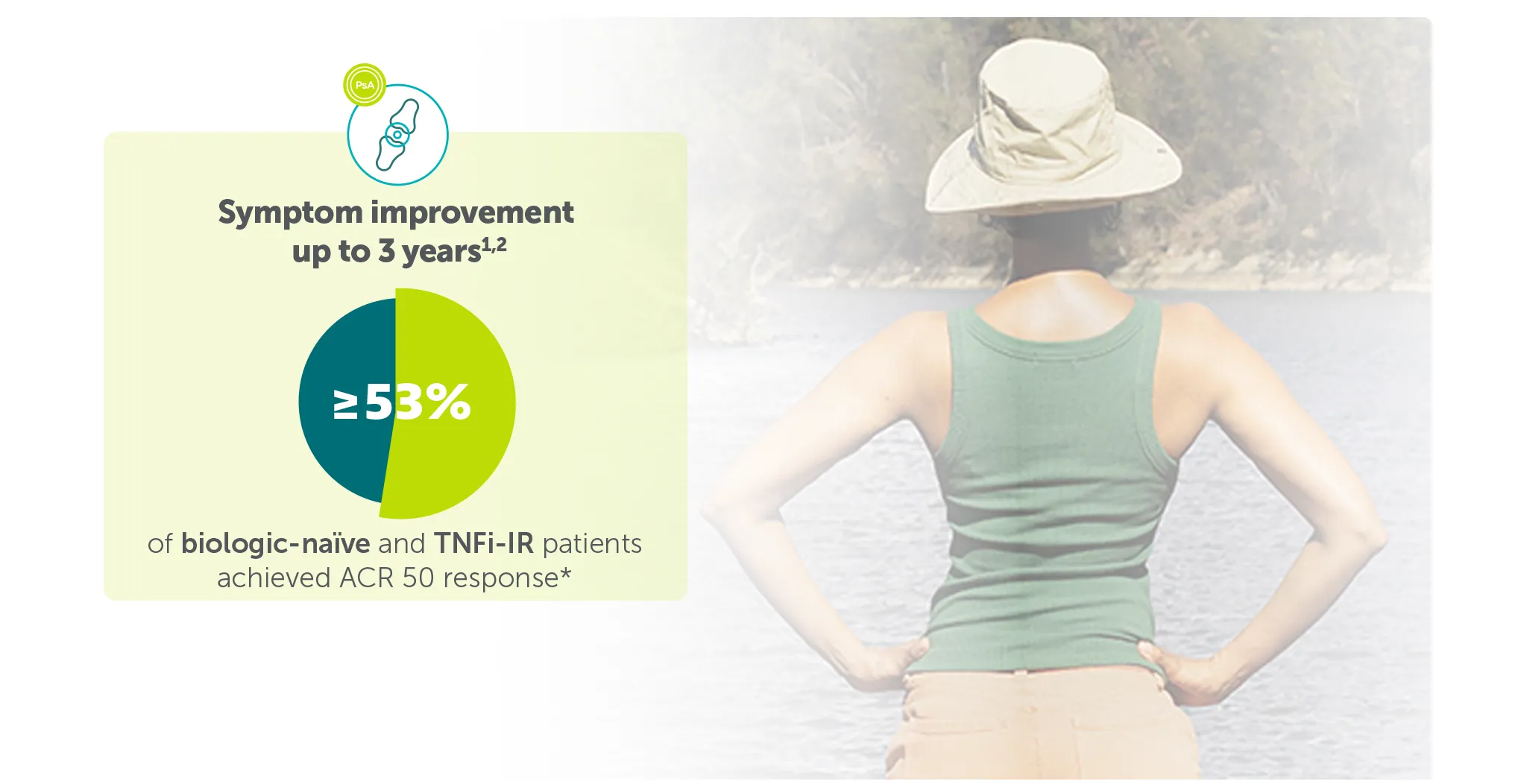
*ACR 50 achieved by 43.4% (116/267) TNFI-IR and 43.9% (189/431) biologic-naïve patients with PsA at Week 16 (primary endpoint in BE COMPLETE and BE OPTIMAL; vs 6.8% (9/133) and 10.0% (28/281) with placebo, respectively; p<0.001), 4–6 51.7% (138/267) and 54.5% (235/431) at Week 52, respectively (NRI analysis).7,8 ACR 50 was achieved by 54.2% (n=400) of TNFI-IR patients at Week 100 and 55.0% (n=712) of biologic-naïve patients at Week 104; 55.2% (n=400) at Week 156 and 53.2% (n=712) at Week 160, respectively, in the BE VITAL OLE in the BIMZELX treatment arms (mNRI analysis).1,2
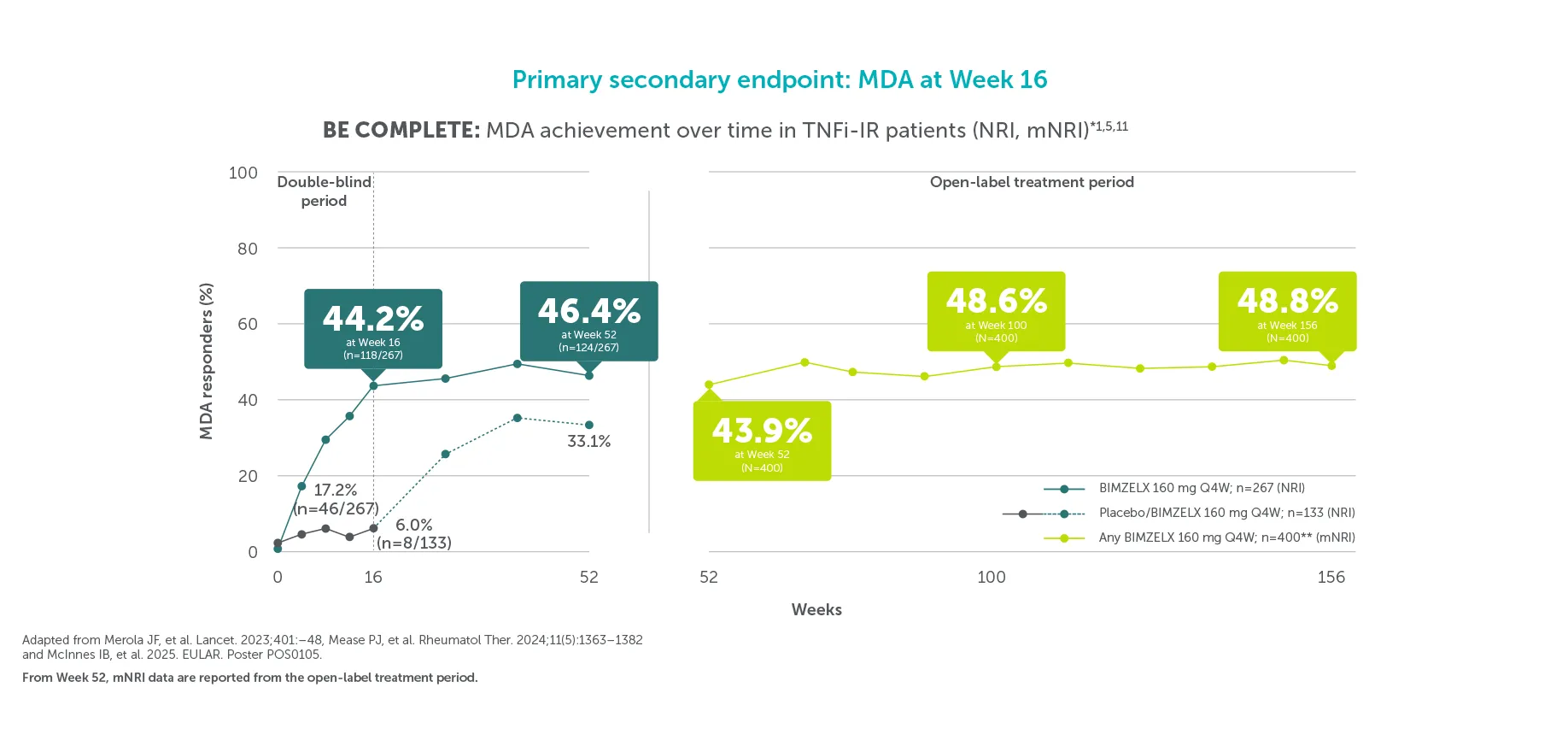
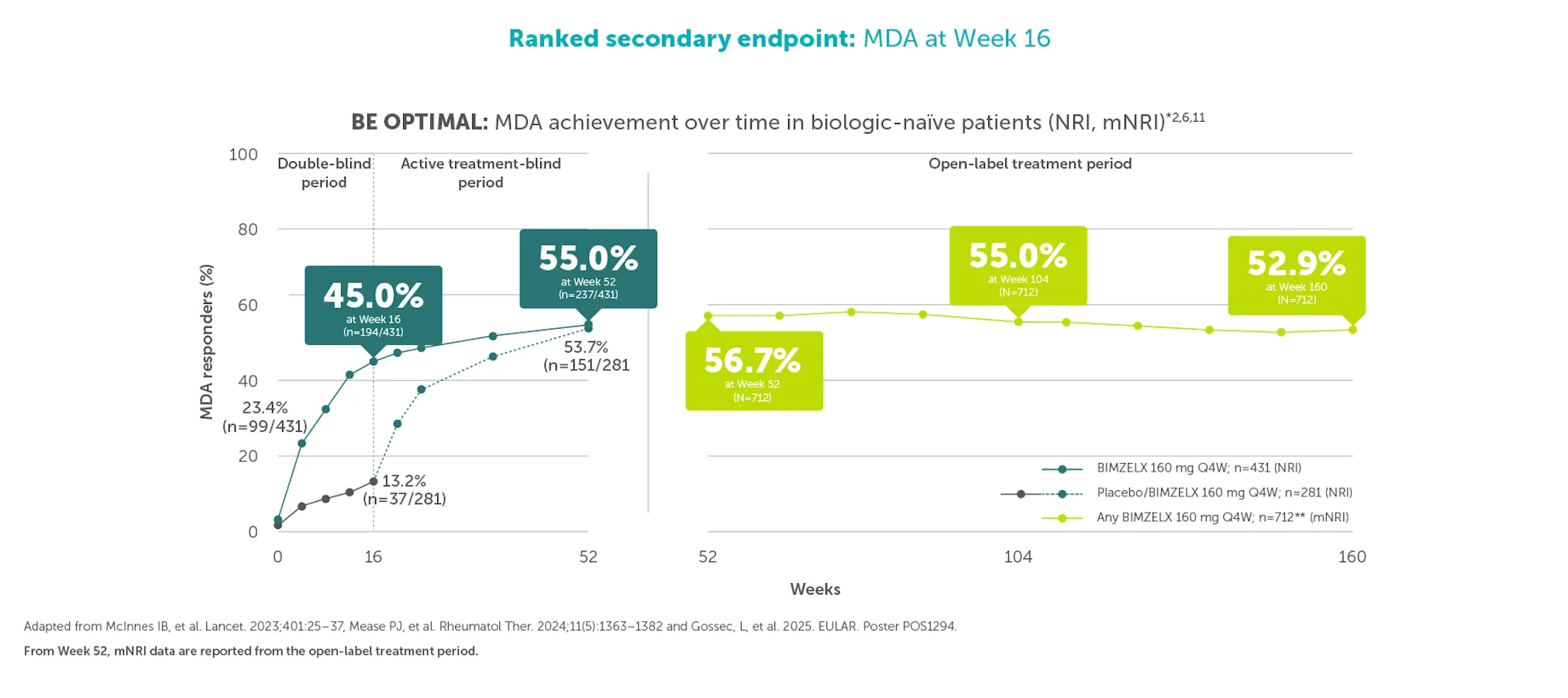
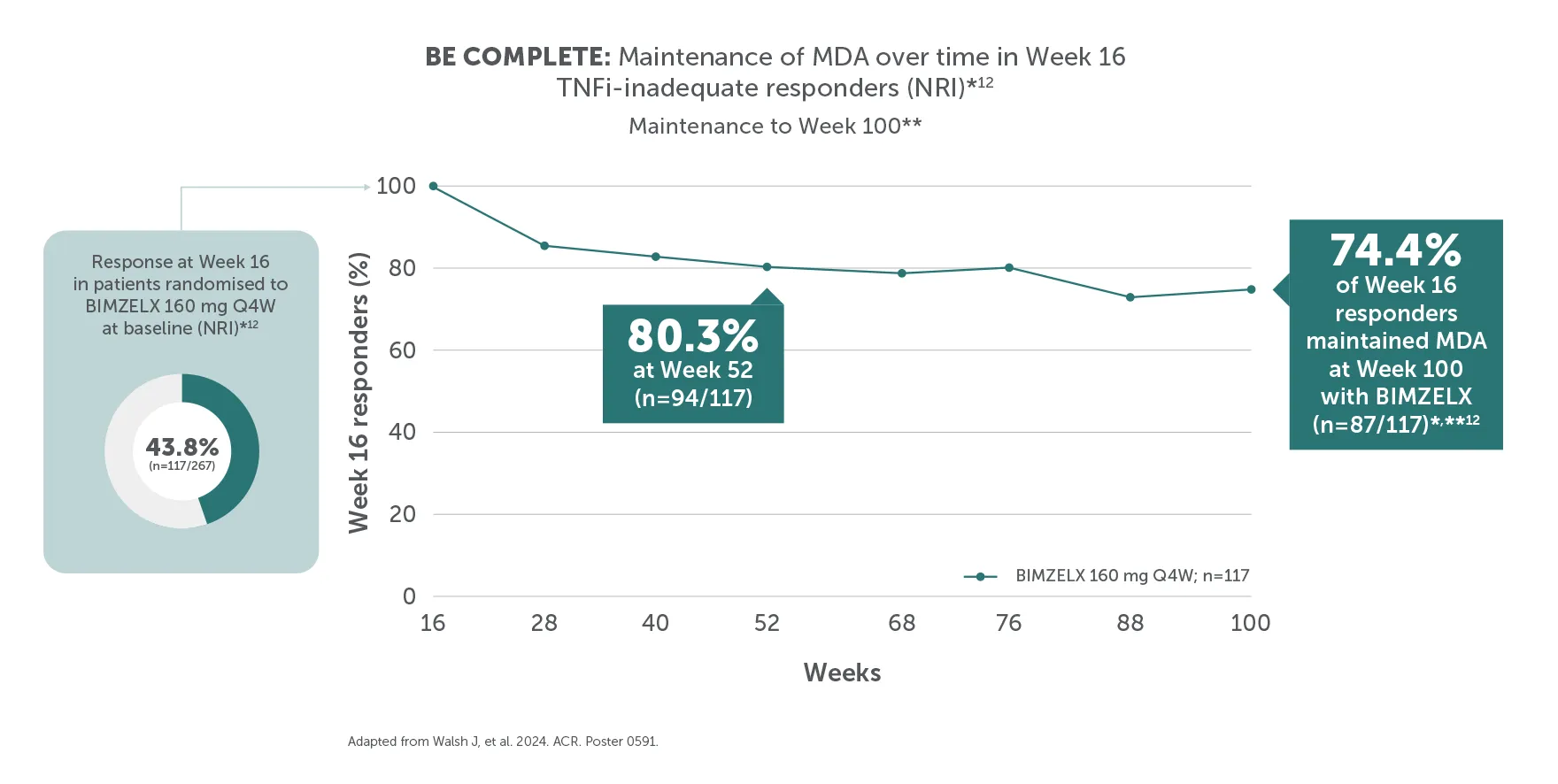
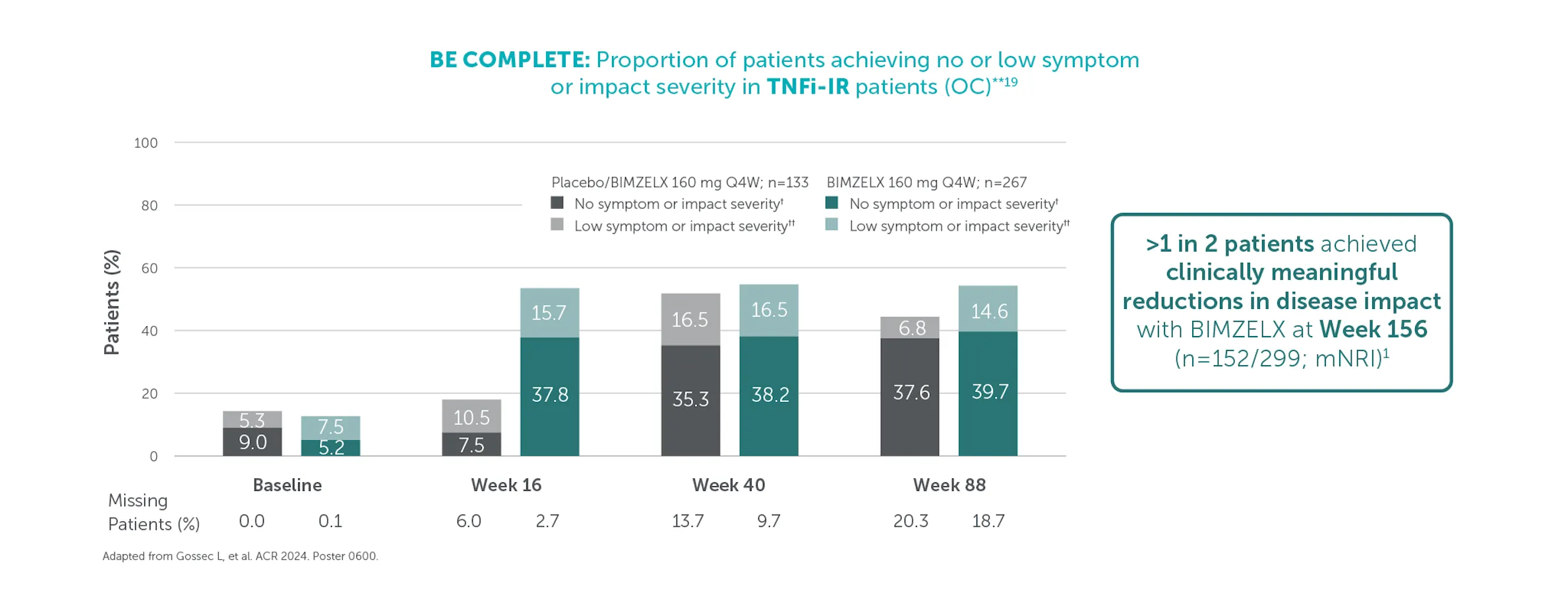
**In BE COMPLETE and the BE VITAL OLE, MDA was achieved by 44.2% (118/267) of TNFI-IR patients in the BIMZELX treatment arm at Week 16 (ranked secondary endpoint; NRI analysis).5 In the open-label treatment period, MDA was achieved by 43.9% of TNFI-IR BIMZELX-treated patients at Week 52, 48.6% at Week 100 and 48.8% at Week 156, respectively (mNRI analysis; n=400).1 In BE OPTIMAL and the BE VITAL OLE, MDA was achieved by 45.0% (194/431) of biologic-naïve patients in the BIMZELX treatment arm at Week 16 (ranked secondary endpoint; NRI analysis).6 In the open-label treatment period, MDA was achieved by 56.7% of biologic-naïve BIMZELX-treated patients at Week 52, 55.0% at Week 104 and 52.9% at Week 160 (mNRI analysis; n=712).2
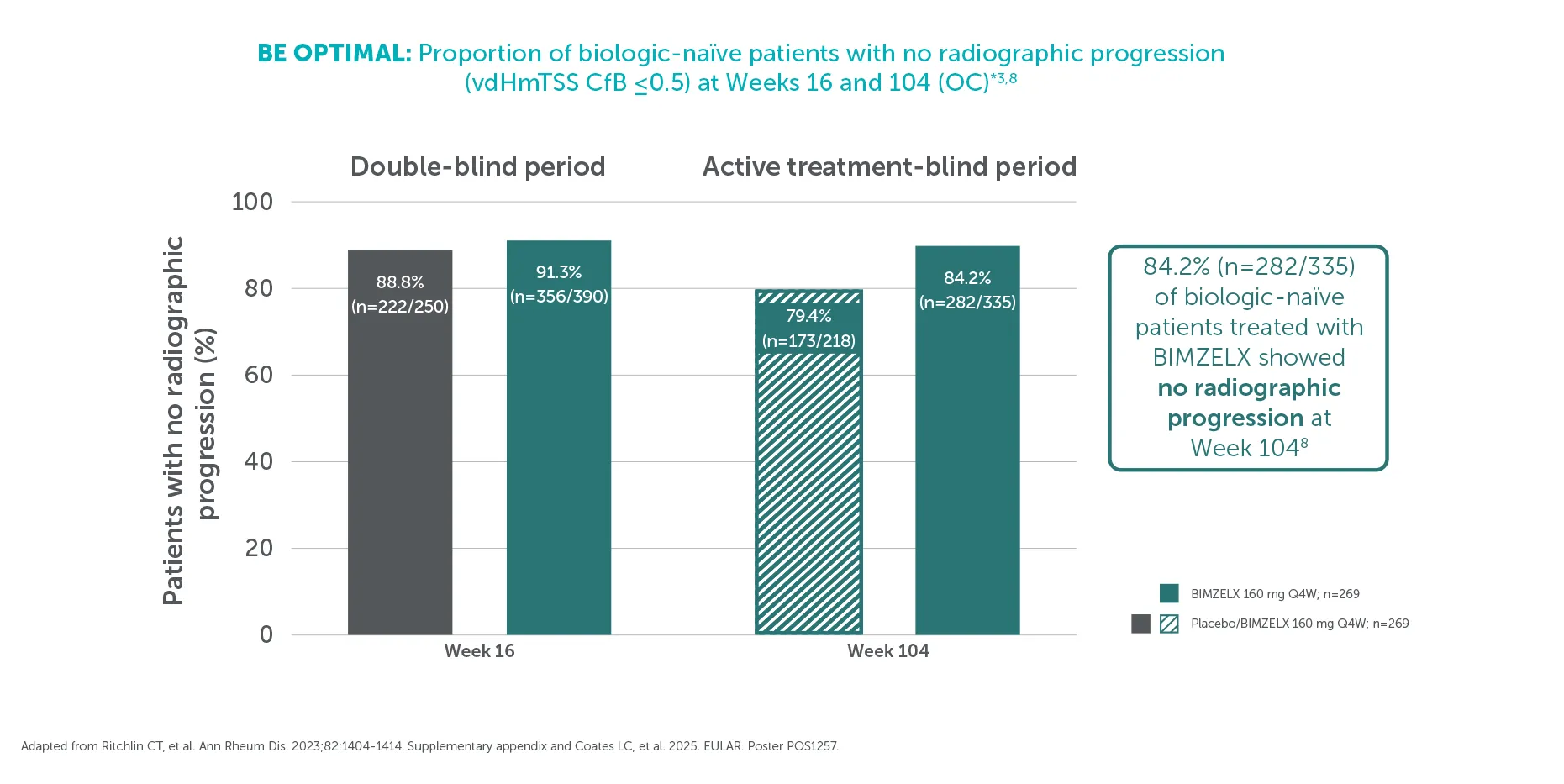
†At Week 104, 84.2% (282/335) of biologic-naïve patients in BE OPTIMAL showed no structural damage progression (defined as vdHmTSS change from baseline ≤0.5) with BIMZELX vs 79.4% (173/218) in the group that switched from placebo to BIMZELX at Week 16 (OC analysis).3
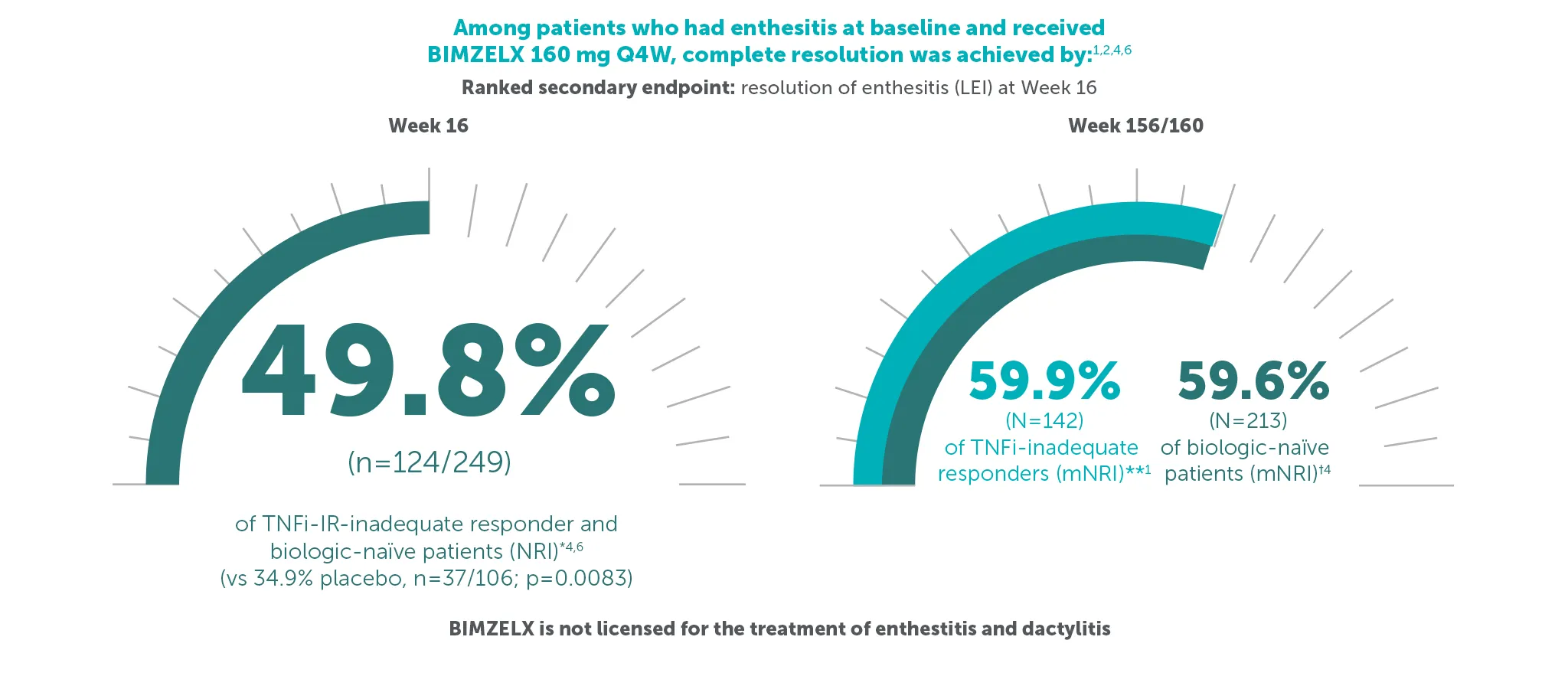
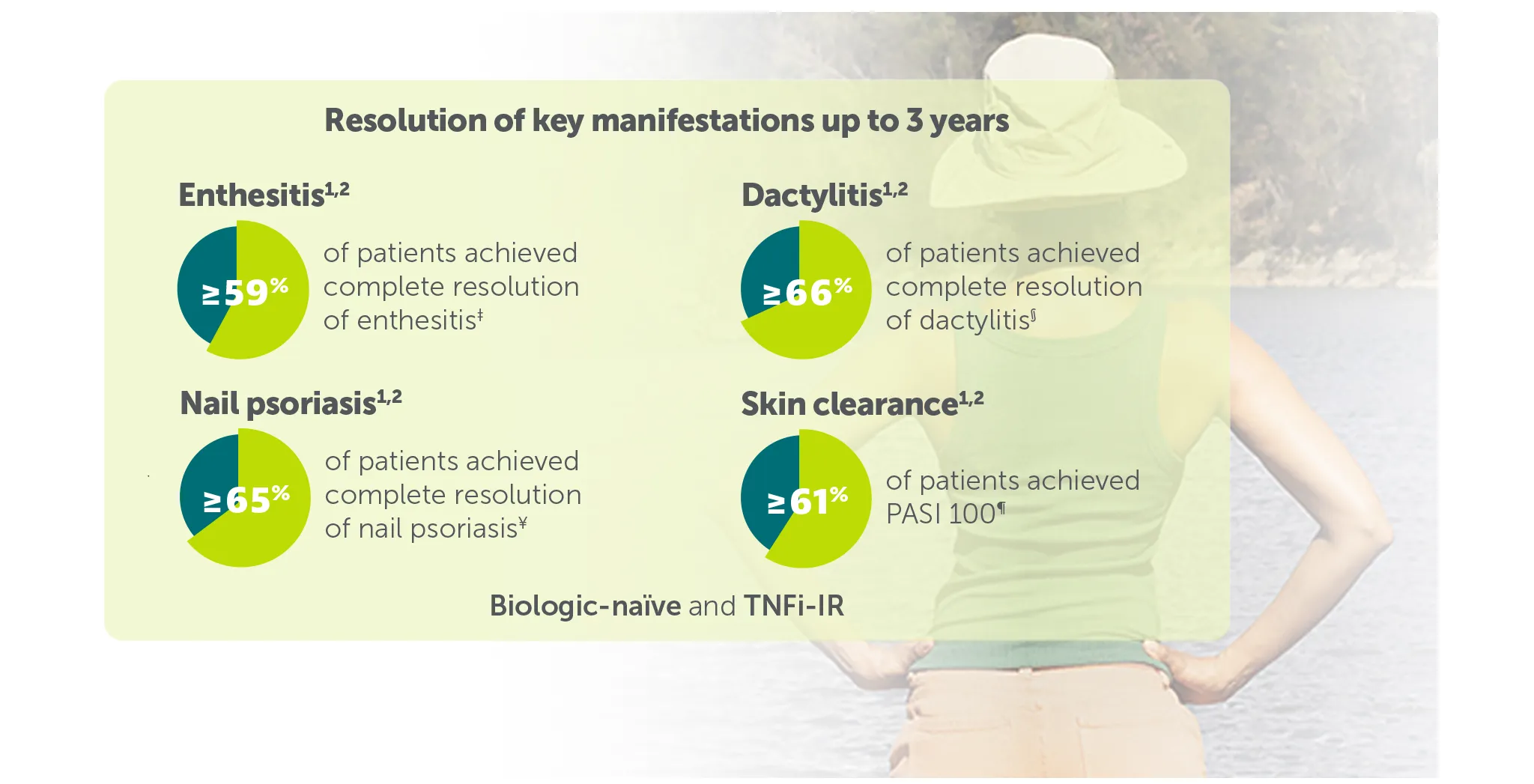
‡59.9% (n=142) of TNFI-IR patients and 59.6% (n=213) of biologic-naïve patients in the BE VITAL OLE of BE COMPLETE and BE OPTIMAL achieved complete resolution of enthesitis (LEI=0) at Weeks 156/160, respectively (additional efficacy outcome in BE COMPLETE and ranked secondary endpoint at Week 16 in BE OPTIMAL, mNRI analysis).1,2,6,7
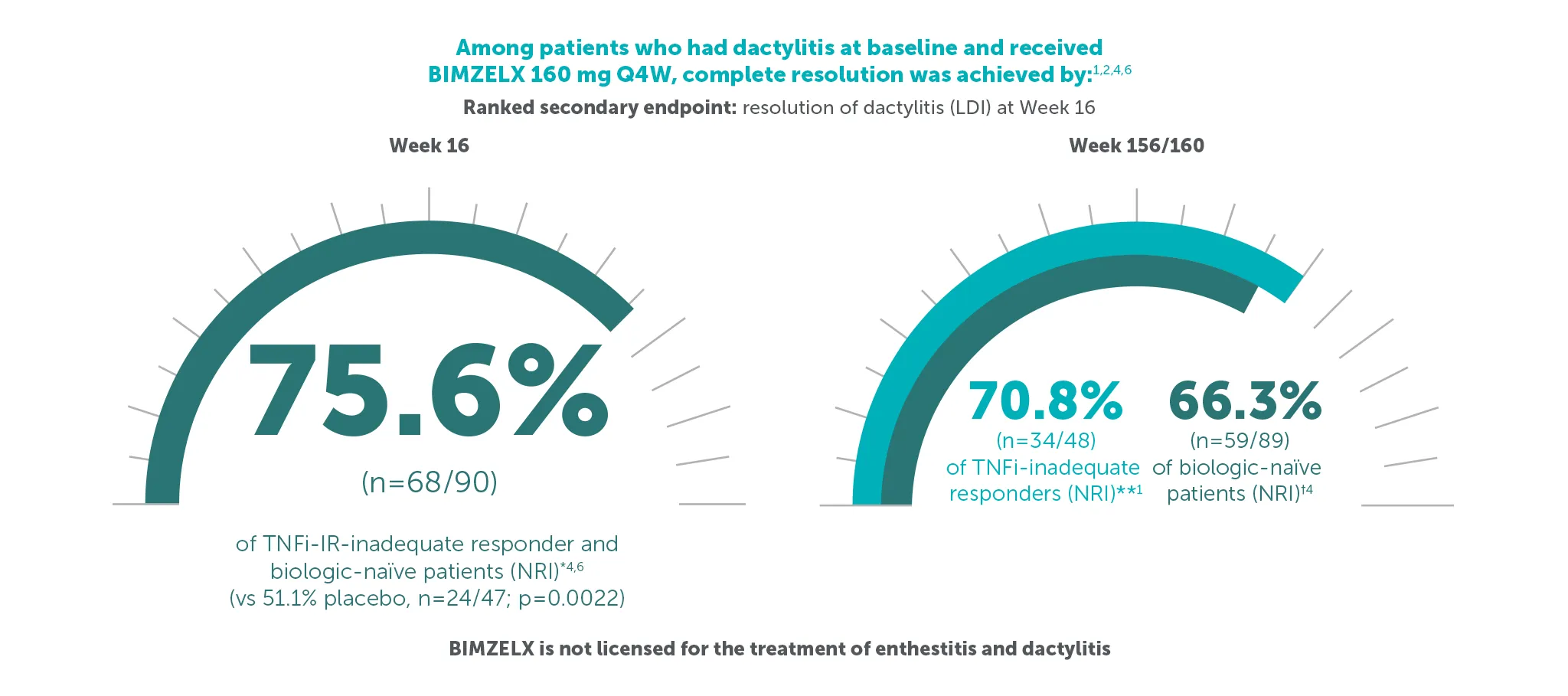
§70.8% (n=48) of TNFI-IR patients and 66.3% (n=89) of biologic-naïve patients in the BE VITAL OLE of BE COMPLETE and BE OPTIMAL achieved complete resolution of dactylitis (LDI=0) at Weeks 156/160, respectively (additional efficacy outcome in BE COMPLETE and ranked secondary endpoint at Week 16 in BE OPTIMAL, NRI analysis). 1,2,6,7
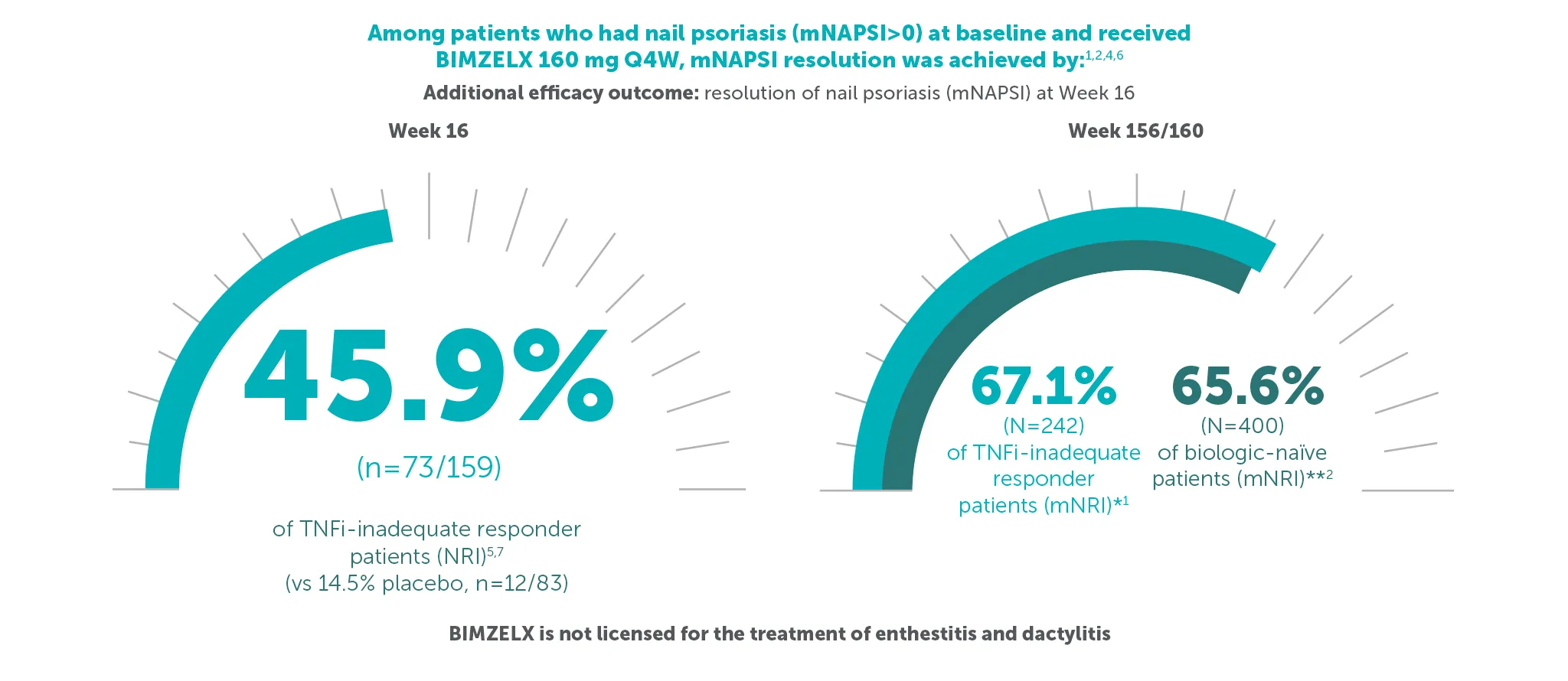
¥67.1% (n=242) of TNFI-IR patients and 65.6% (n=400) of biologic-naïve patients in the BE VITAL OLE of BE COMPLETE and BE OPTIMAL achieved complete resolution of nail psoriasis (mNAPSI=0) at Weeks 156/160, respectively (additional efficacy outcome in BE COMPLETE and additional efficacy variable at Week 16 in BE OPTIMAL, mNRI analysis).1,2,6,7
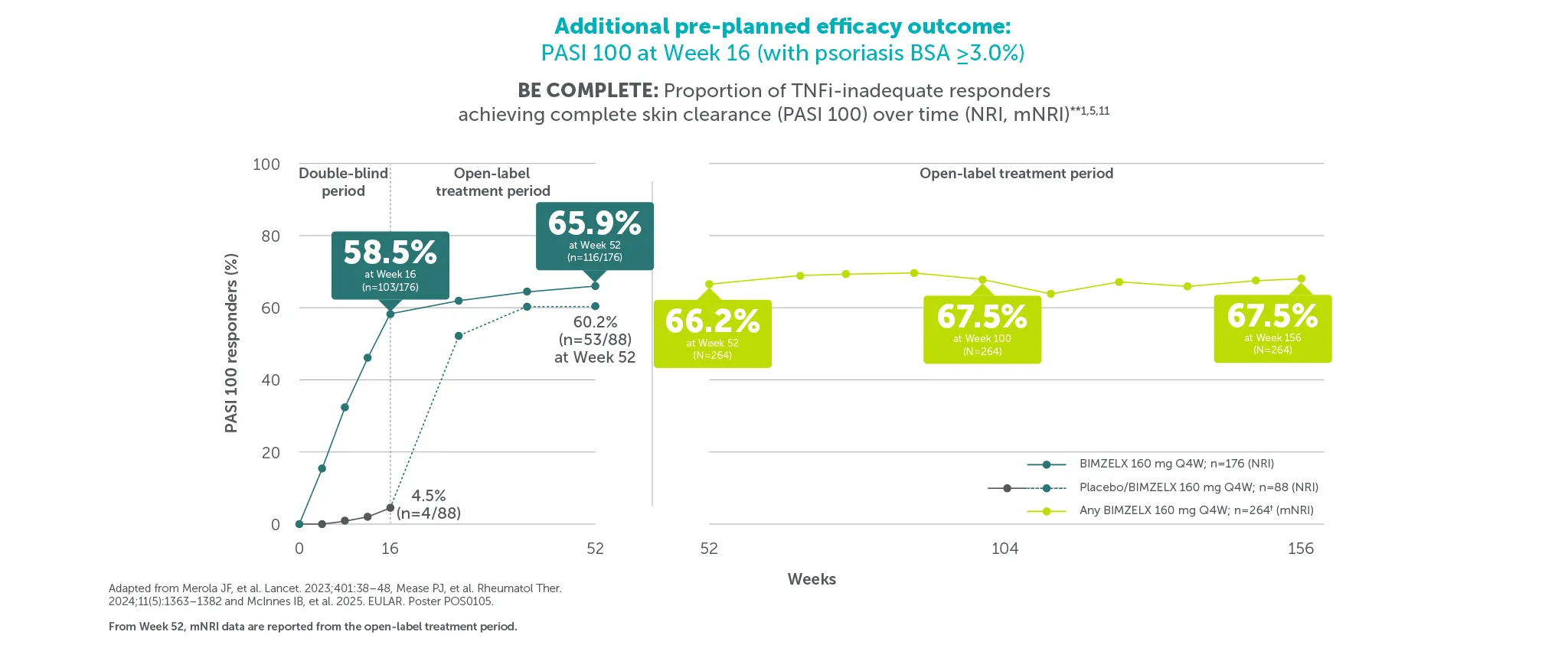
¶58.5% (103/176) and 47.5% (103/217) of patients achieved PASI 100 at Week 16 (vs 4.5% (4/88) and 2.1% (3/140) with placebo) in BE COMPLETE and BE OPTIMAL, respectively (additional pre-planned efficacy outcome at Week 16, NRI analysis).5,6 67.5% (n=264) of TNFI-IR patients and 61.9% (n=357) of biologic-naïve BIMZELX-treated patients achieved PASI 100 at Week 156/160 in the BE VITAL OLE (mNRI analysis).1,2 PASI response in patients with psoriasis involving at least 3% BSA at baseline.1,2
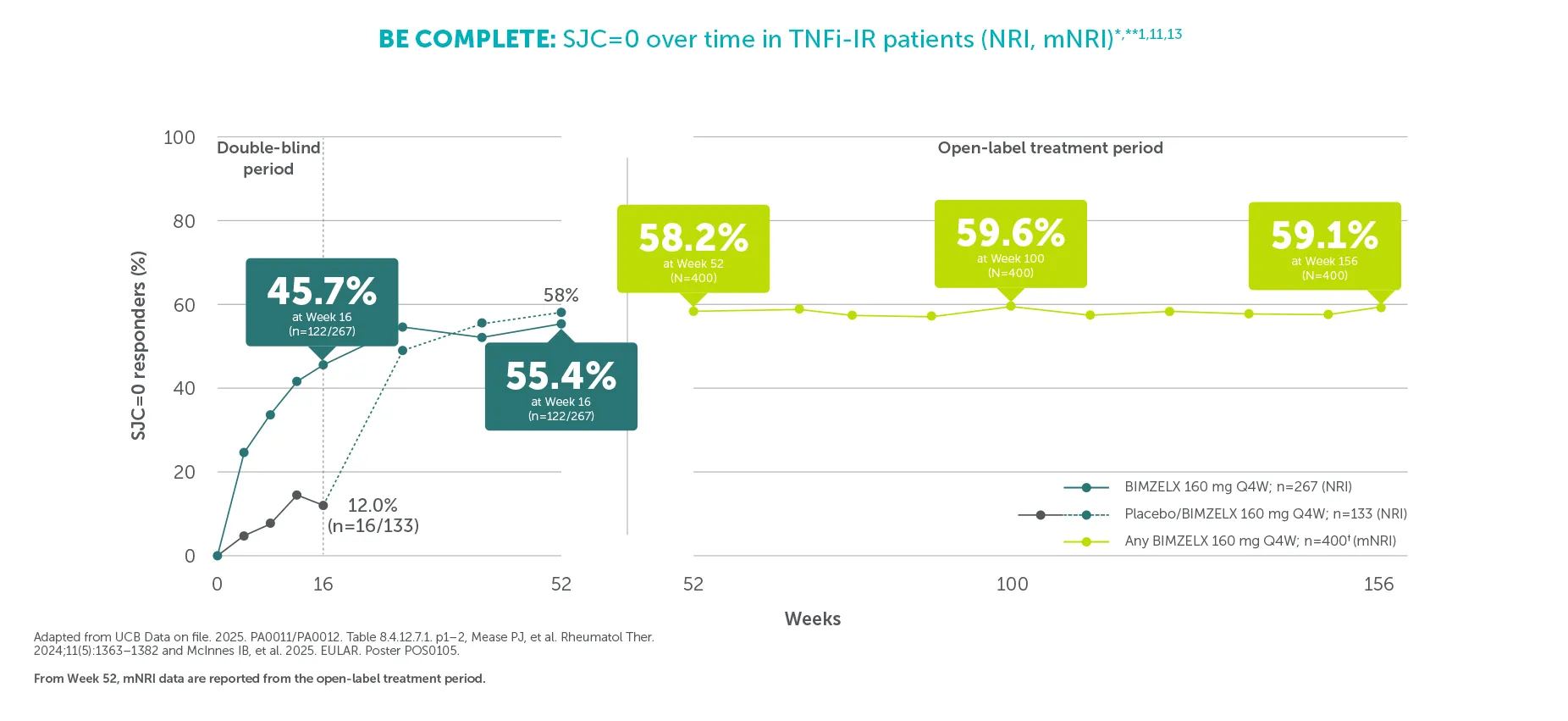
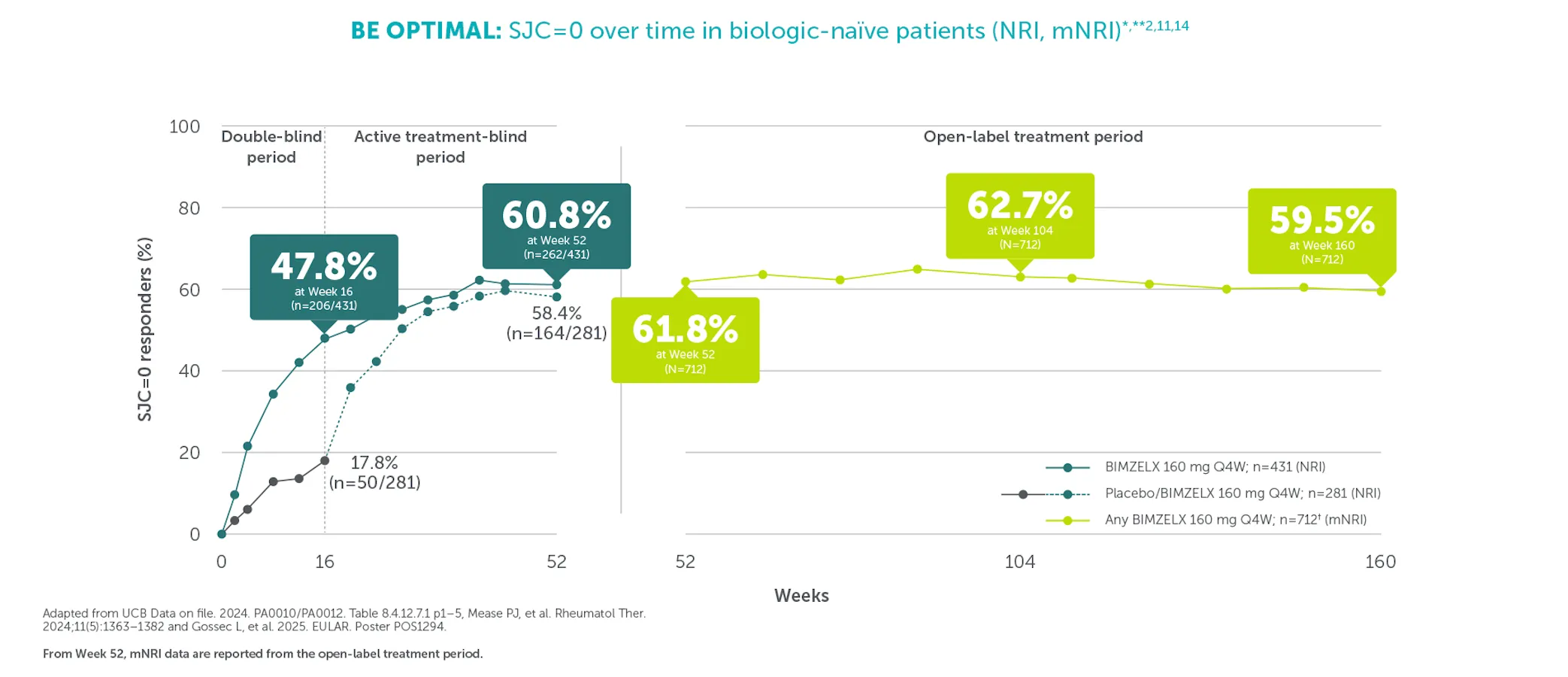
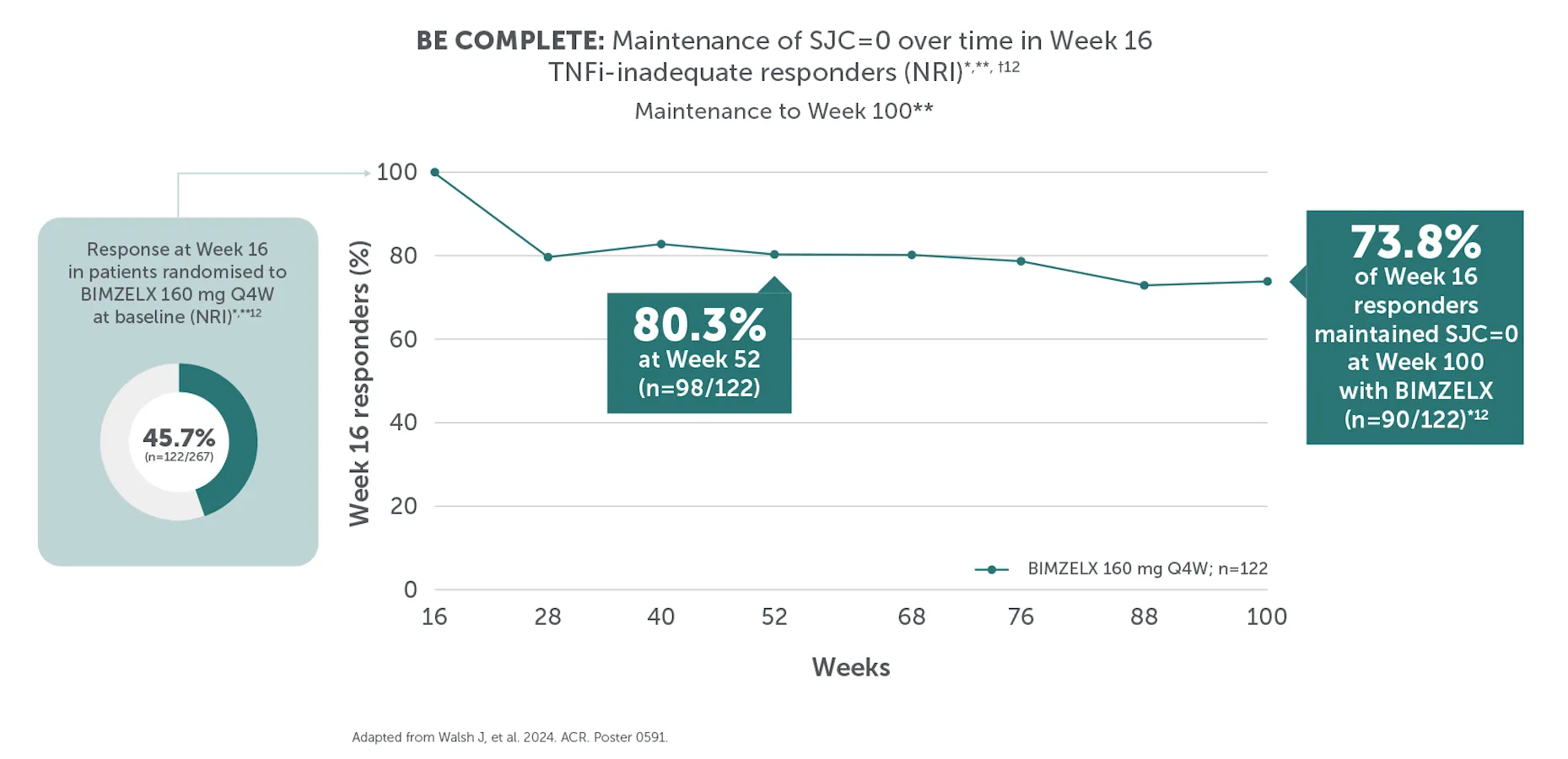
#In the BE VITAL OLE of BE COMPLETE and BE OPTIMAL, SJC=0 was achieved by 59.1% (n=400) of TNFi-IR patients at Week 156 and 59.5% (n=712) of biologic-naïve patients at Week 160 in the BIMZELX treatment arms, respectively (mNRI analysis).1,2 TJC=0 was achieved by 33.0% (n=400) of TNFI-IR patients at Week 156 and 36.9% (n=712) of biologic-naïve patients at Week 160 in the BIMZELX treatment arms, respectively (mNRI).1,2
†† In BE VITAL OLE of BE COMPLETE and BE OPTIMAL:
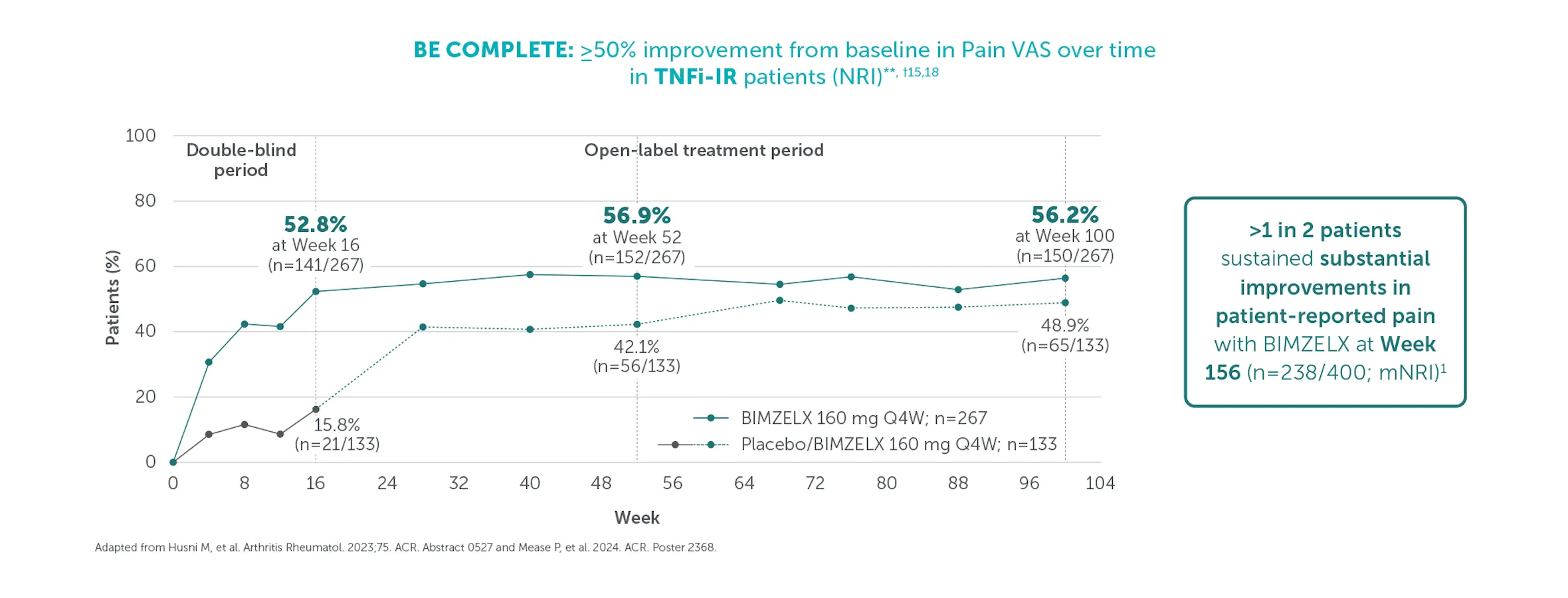
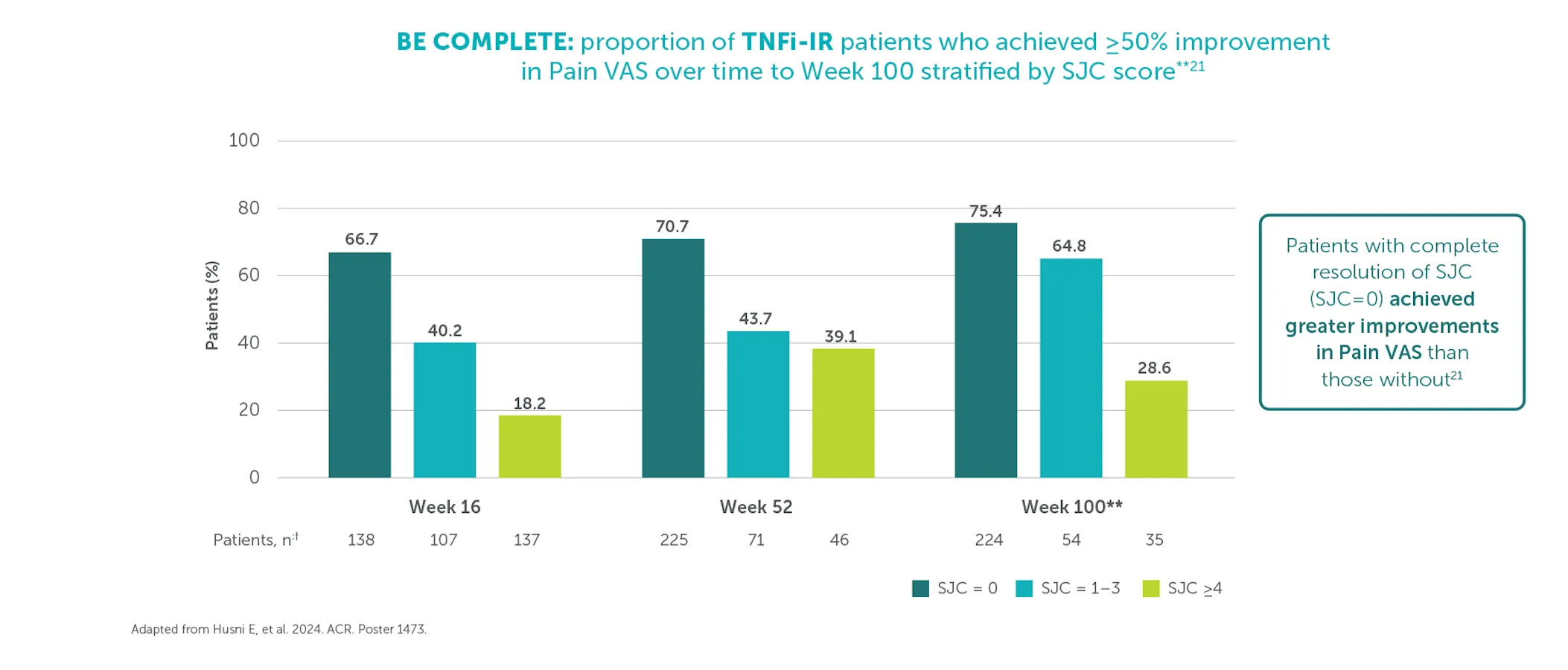
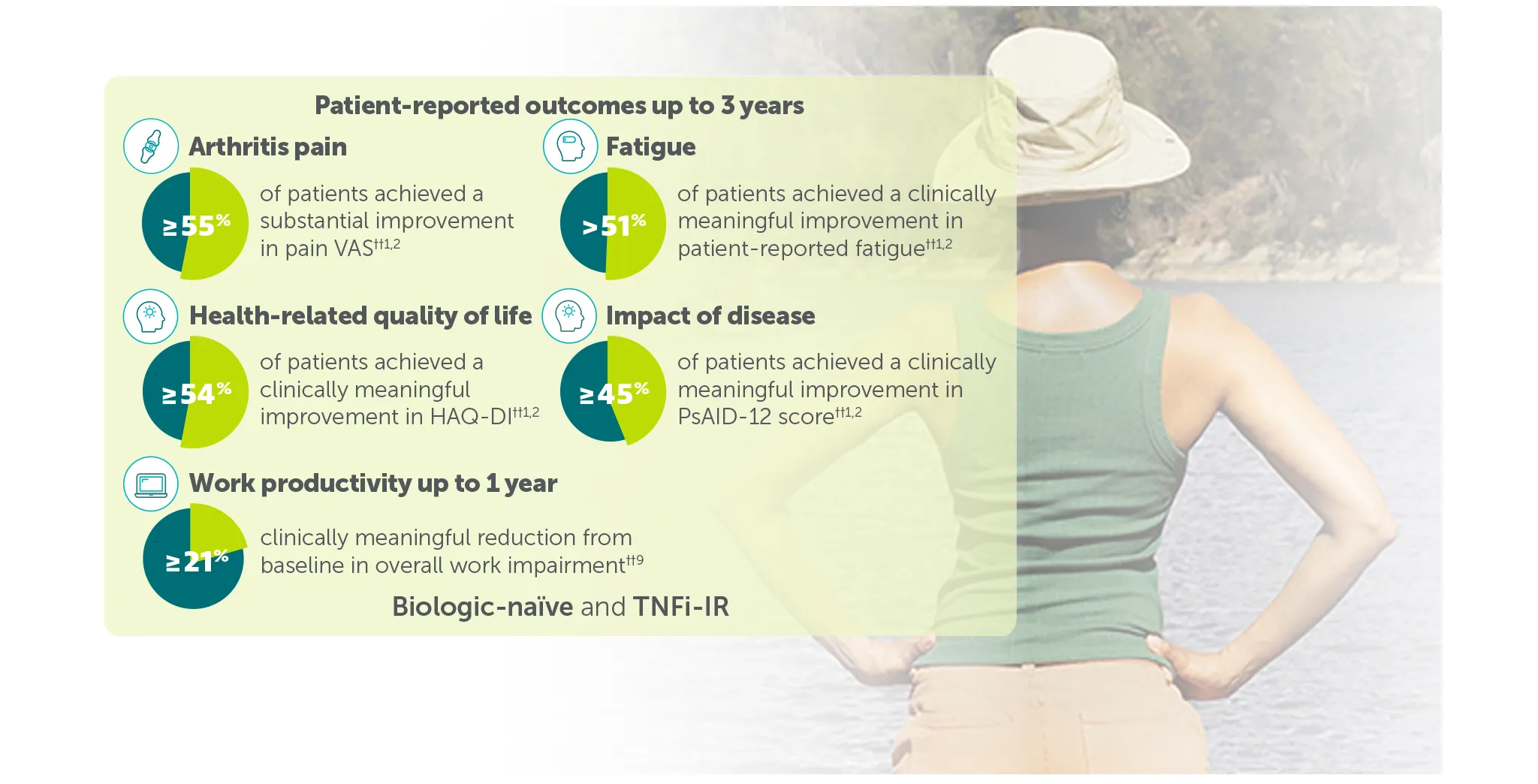
≥50% (substantial) improvement in Pain VAS was achieved by 59.4% (n=400) of TNFI-IR patients and 55.2% (n=712) of biologic-naïve patients at Weeks 156 and 160, respectively (mNRI analysis).1,2
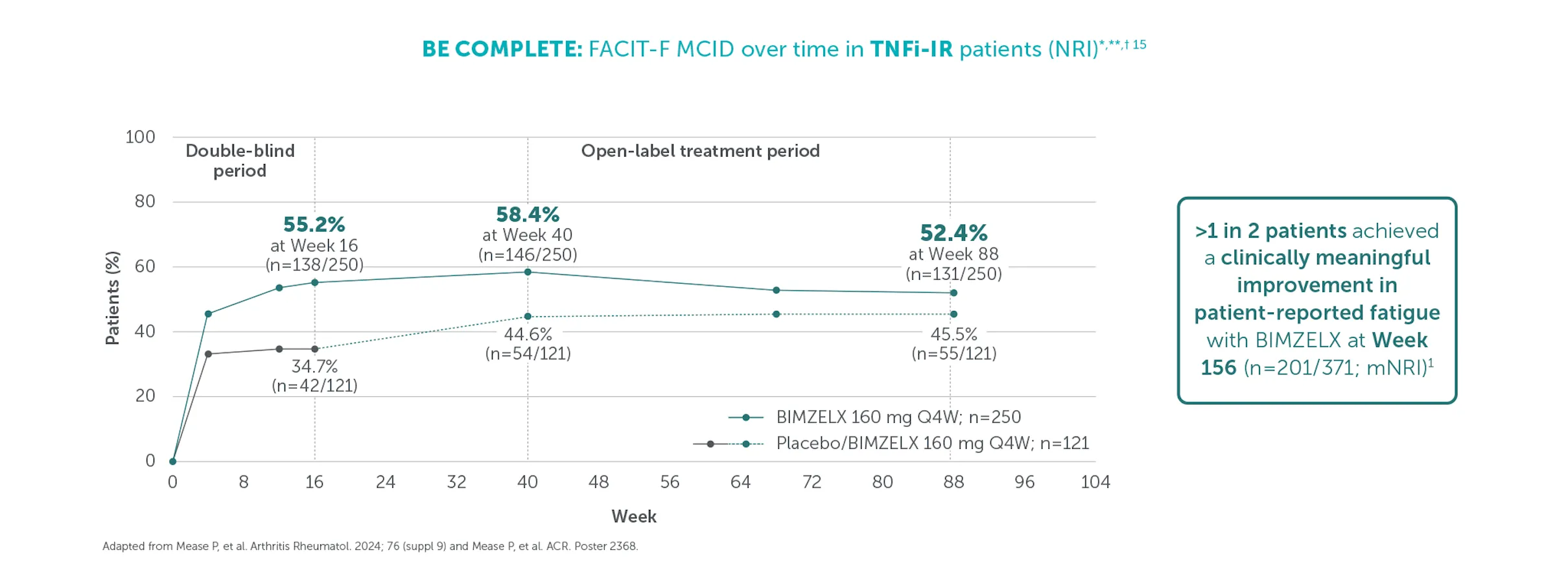
Clinically meaningful improvement in fatigue (FACIT-Fatigue MCID) was achieved by 54.2% (n=371) of TNFI-IR patients and 51.5% (n=643) of biologic-naïve patients at Weeks 156 and 148, respectively (mNRI analysis).1,2 FACIT-Fatigue MCID defined as score increase from baseline ≥4 in patients with FACIT-Fatigue ≤48 at baseline.1,2
Clinically meaningful improvement in HAQ-DI (HAQ-DI MCID) was achieved by 55.7% (n=341) of TNFI-IR patients and 54.4% (n=539) of biologic-naïve patients at Weeks 156 and 160, respectively (mNRI analysis).1,2 HAQ-DI MCID defined as decrease from baseline ≥0.35 in patients with HAQ-DI ≥0.35 at baseline).1,2
50.8% (n=299) of TNFI-IR patients and 45.6% (n=494) of biologic-naïve patients in the BIMZELX treatment arms achieved clinically meaningful reductions in disease impact at Weeks 156 and 148, respectively (mNRI analysis).1,2 Clinically meaningful improvement response: ≥3-point decrease from baseline when respective PsAID-12 score was >3 at baseline.1,2
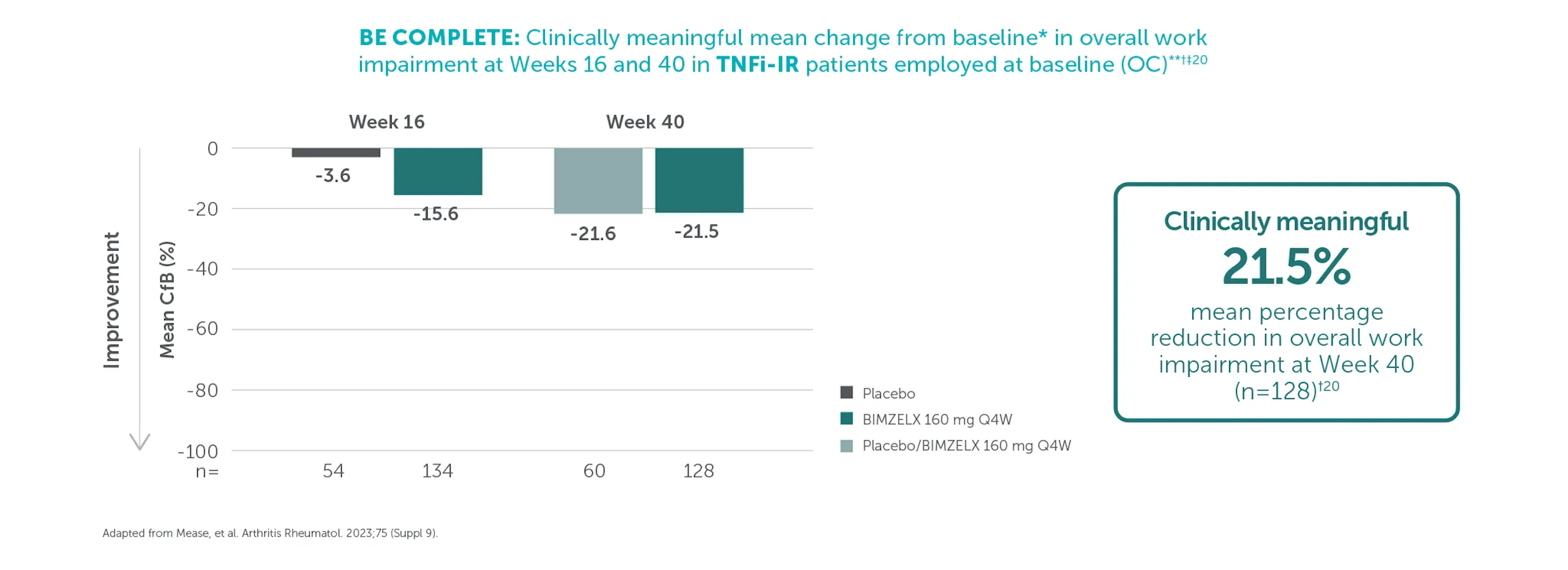
In TNFI-IR patients, mean percentage reduction from baseline in overall work impairment was 21.5% (n=128) at Week 40 in the BIMZELX treatment arm (OC analysis).9 In biologic-naïve patients, mean percentage reduction from baseline in overall work impairment was 21.3% (n=206) at Week 52 in the BIMZELX treatment arm (OC analysis).9 Clinically meaningful improvement in overall work impairment was estimated to be 15%.9,10
The Psoriatic Arthritis Response Criteria (PsARC): A specific composite responder index in PsA4
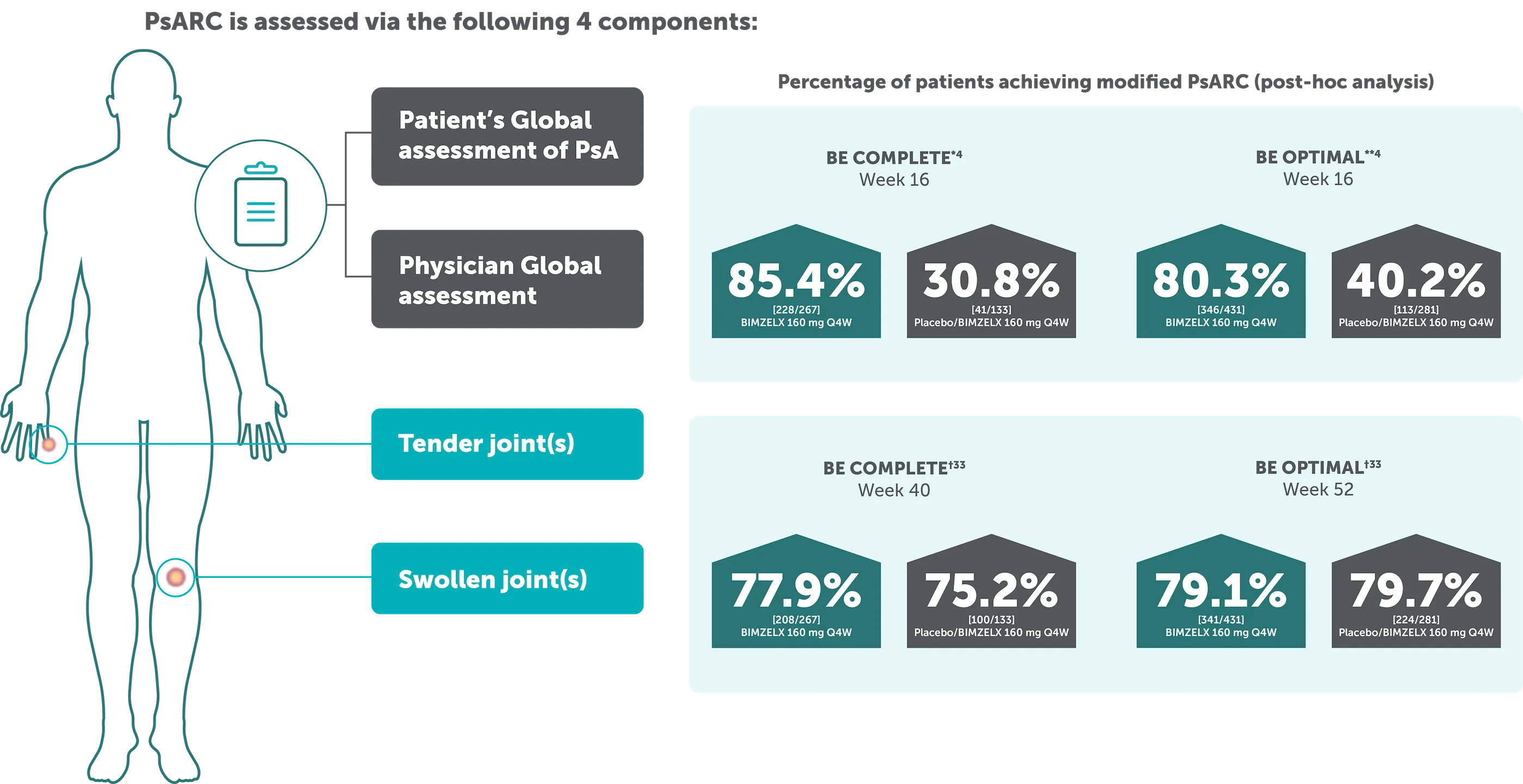
*The proportion of patients achieving modified PsARC at Week 16 was also higher in BE COMPLETE (85.4% vs 30.8% respectively).4 **BE OPTIMAL: The proportion of patients achieving modified PsARC at Week 16 was higher in the bimekizumab-treated patients compared to placebo (80.3% vs 40.2% respectively).4 †79.1% (341/431) of biologic-naïve patients in BE OPTIMAL and 77.9% (208/267) of TNF-IR patients in BE COMPLETE achieved PsARC with BIMZELX at Week 52 and at Week 40, respectively (NRI analysis).33
Patient-reported components and clinical measures of MDA over time (NRI)34
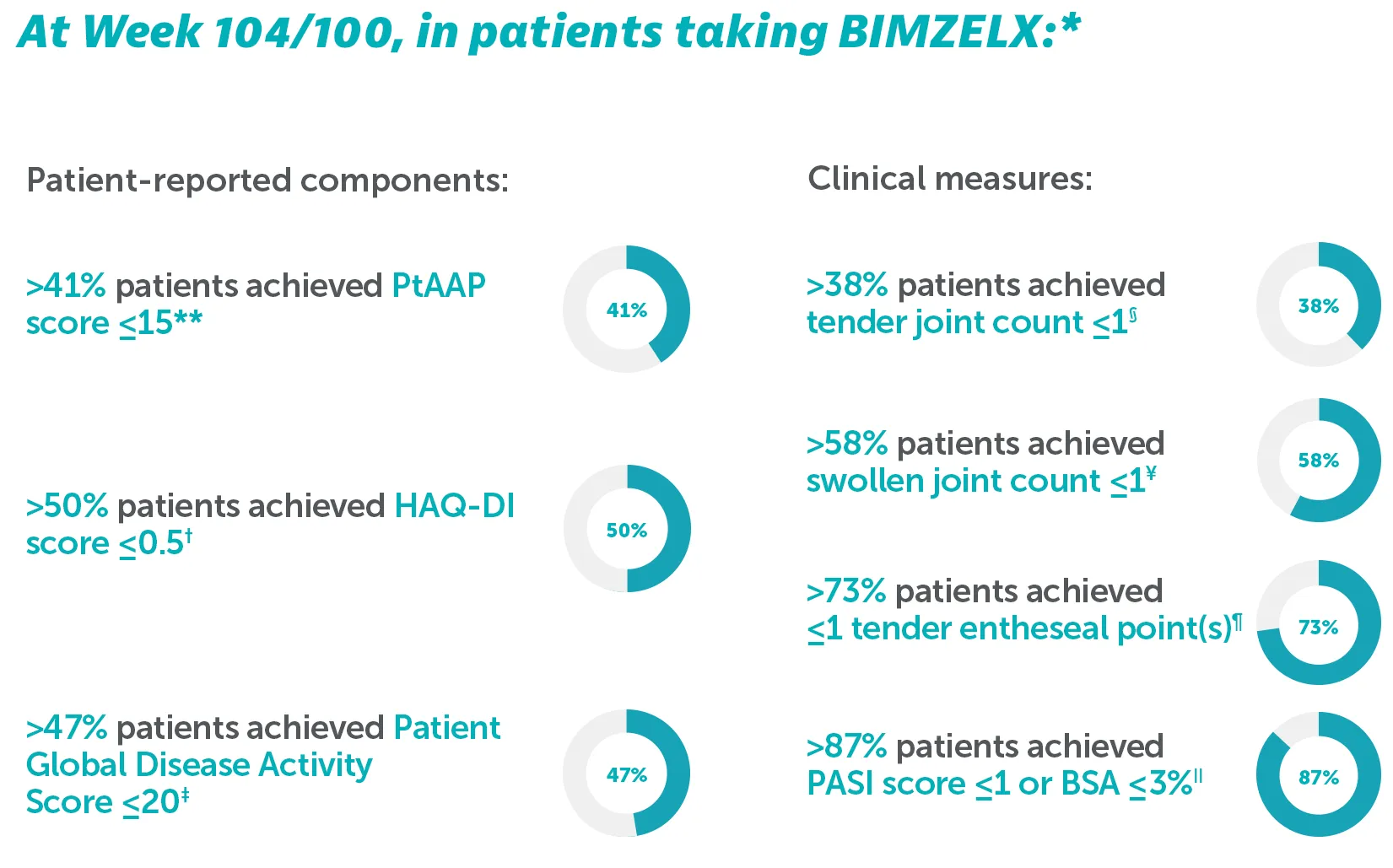
*Outcomes are reported to Week 104 for BE OPTIMAL and Week 100 for BE COMPLETE as some patients had not yet completed their Week 104 visit at time of reporting.34
**In BE OPTIMAL at Week 104, PtAAP ≤15 was achieved by 45.7% (n=197/431) of biologic-naïve patients; In BE COMPLETE at Week 100, PtAAP ≤15 was achieved by 41.9% (n=112/267) of TNF-IR patients.34 †In BE OPTIMAL at Week 104, HAQ-DI ≤0.5 was achieved by 54.1% (n=233/431) of biologic-naïve patients; In BE COMPLETE at Week 100, HAQ-DI ≤0.5 was achieved by 50.6% (n=136/267) of TNFI-IR patients.34 ‡In BE OPTIMAL at Week 104, Patient Global Disease Activity Score ≤20 was achieved by 51.0% (n=220/431) of biologic-naïve patients; In BE COMPLETE at Week 100, Patient Global Disease Activity Score ≤20 was achieved by 47.6% (n=127/267) of TNFI-IR patients.34 §In BE OPTIMAL at Week 104, TJC ≤1 was achieved by 46.4% (n=200/431) of biologic-naïve patients; In BE COMPLETE at Week 100, TJC ≤1 was achieved by 38.2% (n=102/267) of TNF-IR patients.34 ɎIn BE OPTIMÁL at Week 104, SJC ≤1 was achieved by 67.7% (n=292/431) of biologic-naïve patients; In BE COMPLETE at Week 100, SJC ≤1 was achieved by 58.8% (n=157/267) of TNF-IR patients.34 ¶In BE OPTIMAL at Week 104, LEI ≤1 achieved by 74.7% (n=318/431) of biologic-naïve patients; In BE COMPLETE at Week 100, LEI ≤1 was achieved by 73.0% (n=195/267) of TNFI-IR patients.34 IIIn BE OPTIMAL at Week 104, PASI ≤1/BSA ≤3% was achieved by 89.3% (n=385/431) of biologic-naïve patients, In BE COMPLETE at Week 100, PASI ≤1/BSA ≤3% was achieved by 87.6% (n=234/267) of TNFI-IR patients.34
SAFETY
BIMZELX was generally well tolerated, with long-term exposure up to 3 years22,23
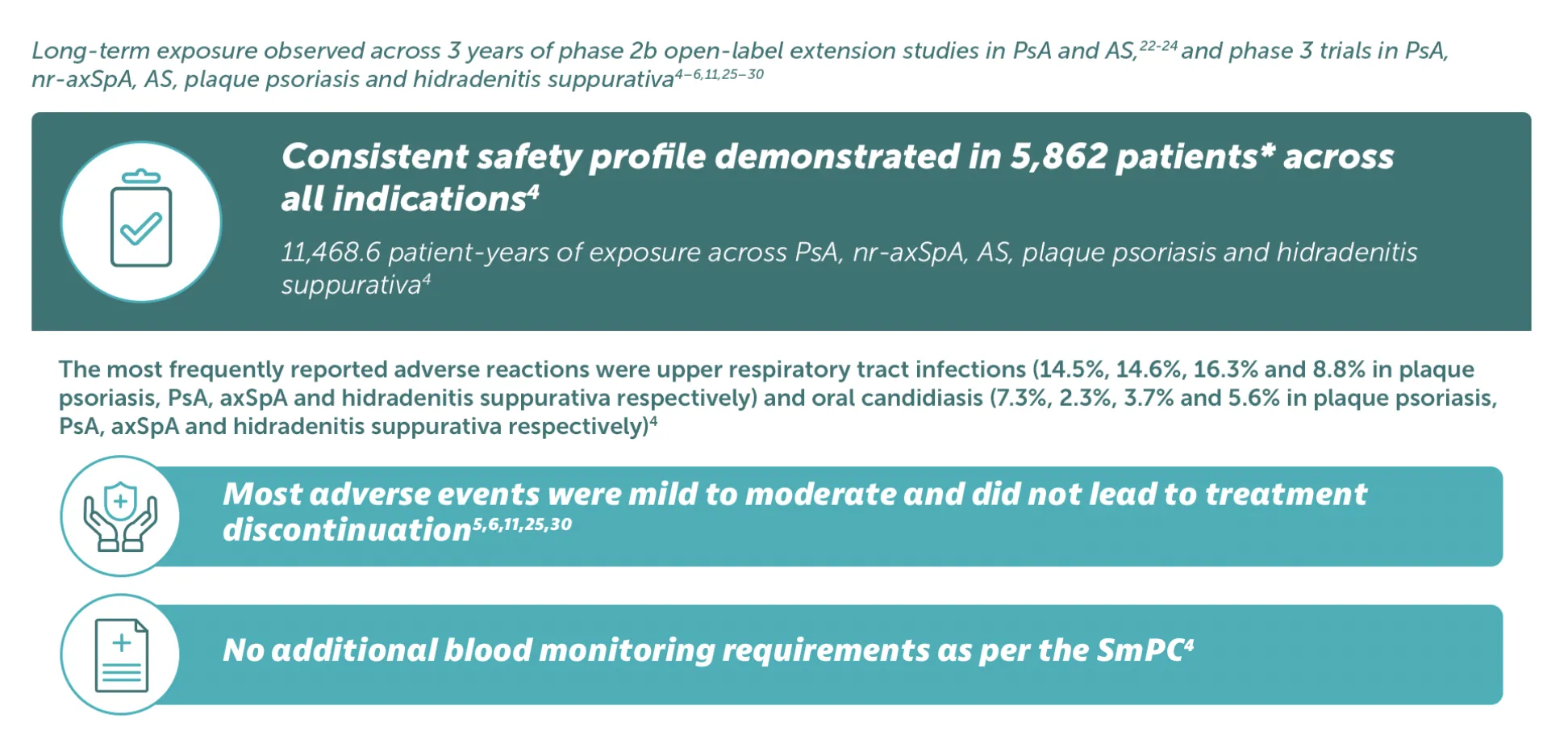
Adverse events: Refer to SmPC for full information. Very common (≥1/10): Upper respiratory tract infection; Common (≥1/100 to< 1/10): Oral candidiasis, tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, dermatitis and eczema, acne, injection site reactions, fatigue; Uncommon (≥1/1,000 to<1/100): Mucosal and cutaneous candidiasis (including oesophageal candidiasis), conjunctivitis, neutropenia, inflammatory bowel disease. *5,862 patients treated in blinded and open-label clinical studies in PsA, nr-axSpA, AS, moderate to severe plaque psoriasis, and moderate to severe HS. Of these, over 4,660 patients were exposed to BIMZELX for at least one year.4
BIMZELX has a consistent long-term safety profile in patient with PsA31
BIMZELX is generally well tolerated in patients with axSpA and PsA as seen by long-term exposure analyses from phase 2b/3 trials in patients with at least 104 weeks of total study participation31
Pooled analysis of patients with PsA:
Overview of TEAEs up to data-cut
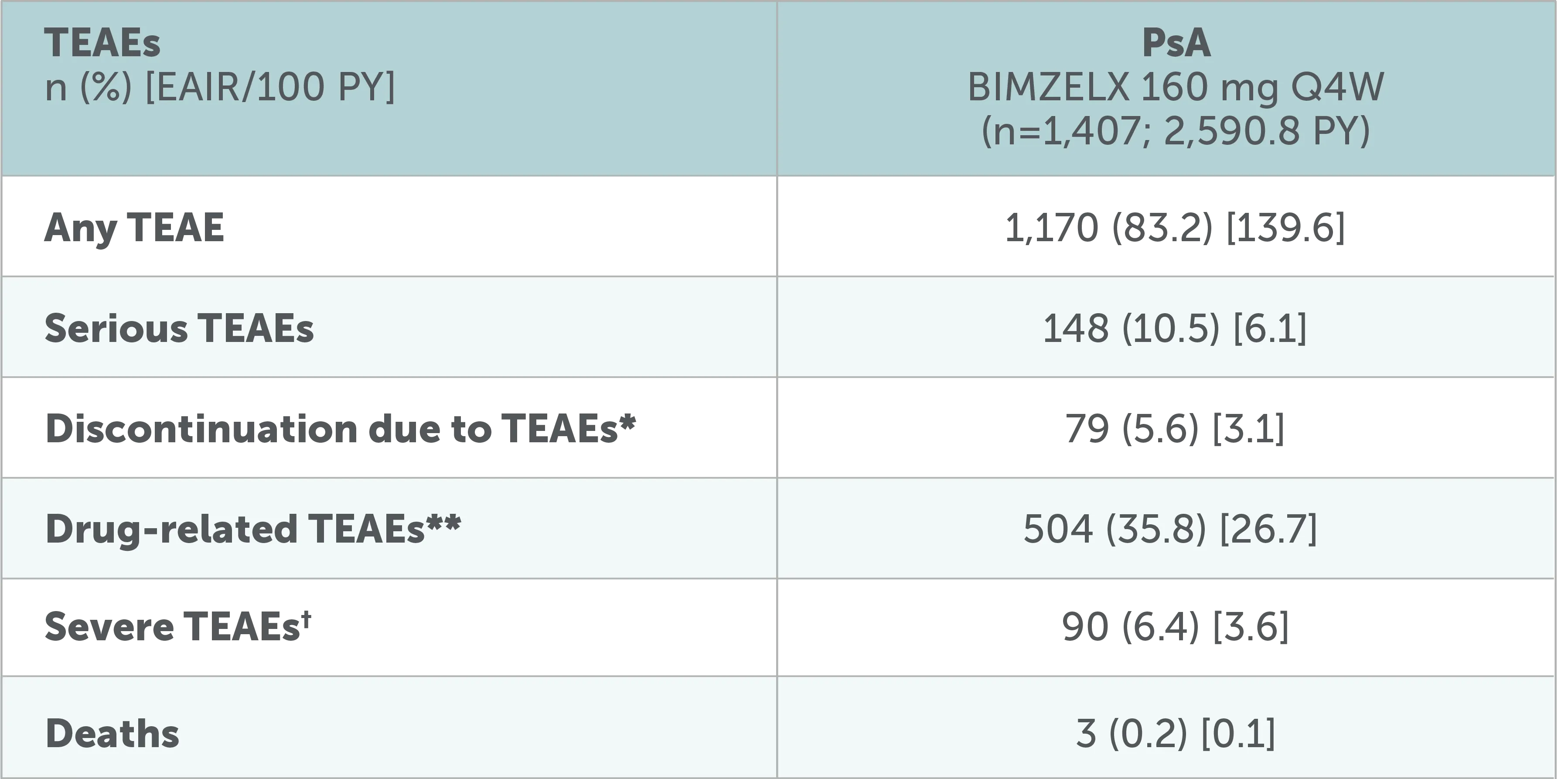
Adapted from Mease P, et al. RMD Open. 2025;11(2):e005026
Data to the July 2022 data-cut shown, including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. After Week 16, all patients were aware that they were receiving active treatment, which may have affected the results. *Including TEAEs leading to death.31 **Per investigator assessment. 31†The intensity of TEAEs was assessed by the investigators as 'mild', 'moderate' or 'severe, independently from seriousness.
Pooled analysis of patients with PsA:
Overview of AEs of special monitoring up to data-cut
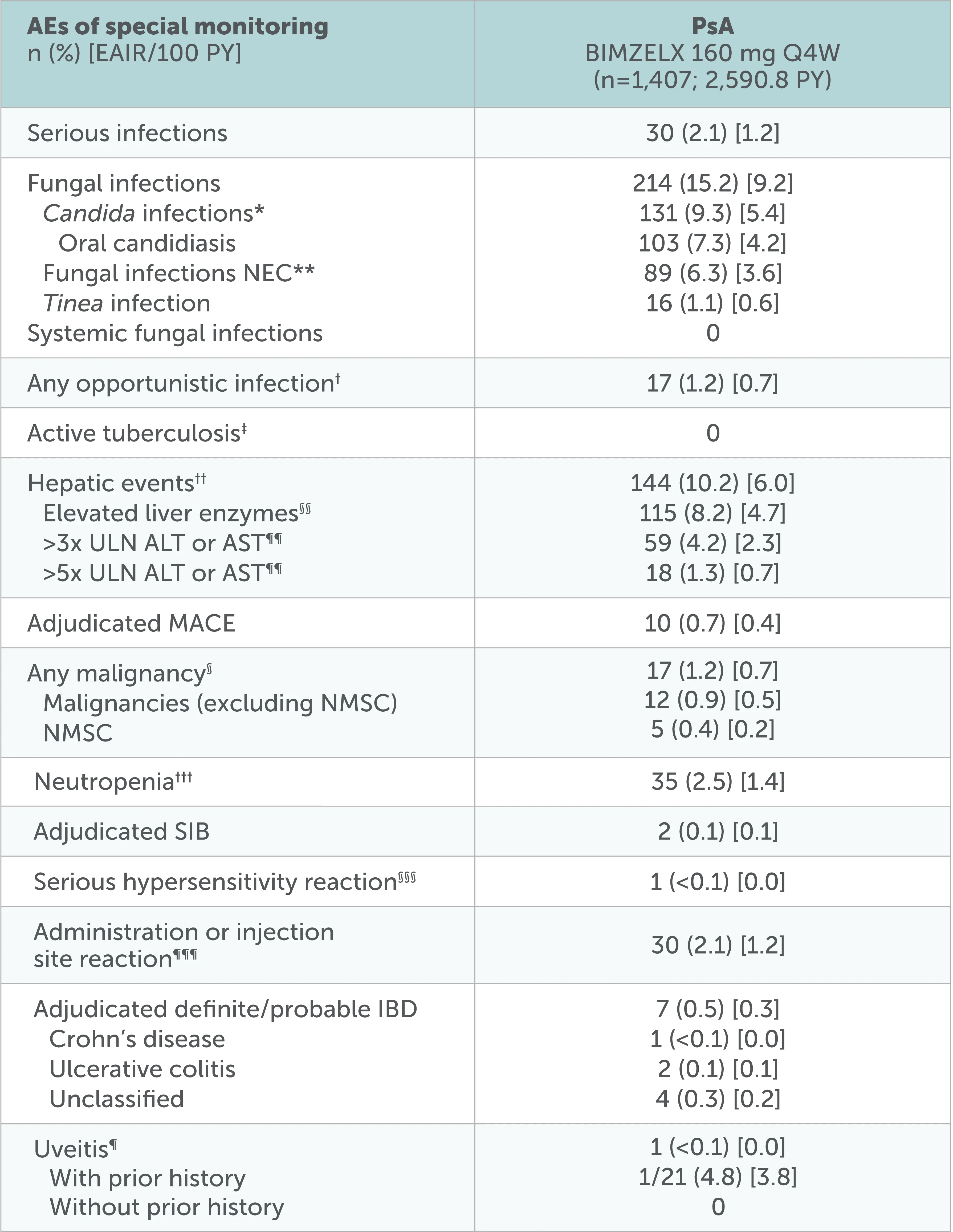
Adapted from Mease P, et al. RMD Open. 2025;11(2):e005026
Data to the July 2022 data-cut shown, including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. After Week 16, all patients were aware that they were receiving active treatment, which may have affected the results. *Shown by MedDRA preferred term in online supplemental table 2.31 **NEC denotes groupings of miscellaneous terms that do not readily fit into other hierarchical classifications within a specific SOC in the MedDRA.31 †Not including one serious case of oropharyngeal candidiasis in a patient with PSA, which was identified as an opportunistic infection after the July 2022 data-cut.31 ‡At baseline, 46 (5.4%) patients with axSpA and 34 (2.4%) patients with PsA had a history of ongoing/latent tuberculosis (phase 2b/3 pool).31 §Identified using the SMQ ‘Malignant tumours’.31 ¶Includes the preferred terms ‘Autoimmune uveitis’, ‘Iridocyclitis', 'Iritis' and 'Uveitis’.31 ††Includes events in the SMQ 'Drug-related hepatic disorders-comprehensive search’ excluding the sub-SMQS 'Liver neoplasms, benign (including cysts and polyps)' and 'Liver neoplasms, malignant and unspecified’.31 §§Elevated liver enzymes include the following preferred terms reported as adverse events: Increased/abnormal levels of ALT, AST, Blood bilirubin, Gamma-glutamyltransferase, Hepatic enzyme, Liver Function test or Transaminases.31 ¶¶n=411 for axSpA; n=1405 for PsA. †††Includes preferred terms identified based on UCB-defined search criteria.31 §§§Hypersensitivity reactions identified via the SMQ ‘Hypersensitivity’.31 ¶¶¶Identified using the HLTS 'Administration site reactions NEC' and 'Injection site reactions'.31
No new safety signals were reported31
The three most common TEAEs were SARS-COV-2 (COVID-19) infection, nasopharyngitis, and upper respiratory tract infection
Incidence rate of oral candidiasis decreased over time and infrequently led to discontinuation
Low incidence of uveitis was seen in patients with PsA across pooled phase 2b/3 trials*32
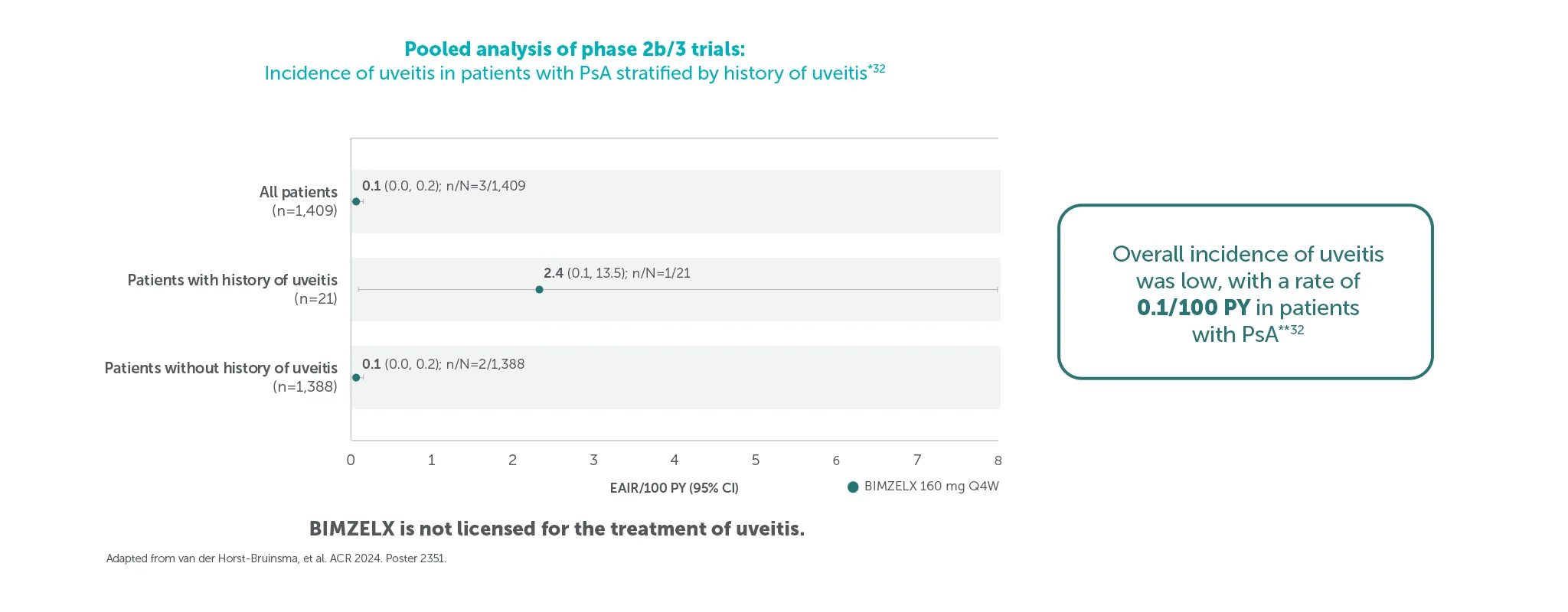
Pooled safety set including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. Uveitis rates and EAIRs/100 PY were reported over median durations of approximately 2.8 years (axSpA) and 2.7 years (PsA); the data cut-off for both patient populations was set at July 2023.32 *Data shown are from the July 2023 data cut.32 Duration by data-cut is varied between patients, depending on feeder study duration and initial randomisation.32 Overall exposure across the pooled phase 2b/3 trials was 3,656 PY.32**All uveitis events were mild or moderate and no events led to treatment discontinuation.32
HOW TO USE
BIMZELX dosing regimen4
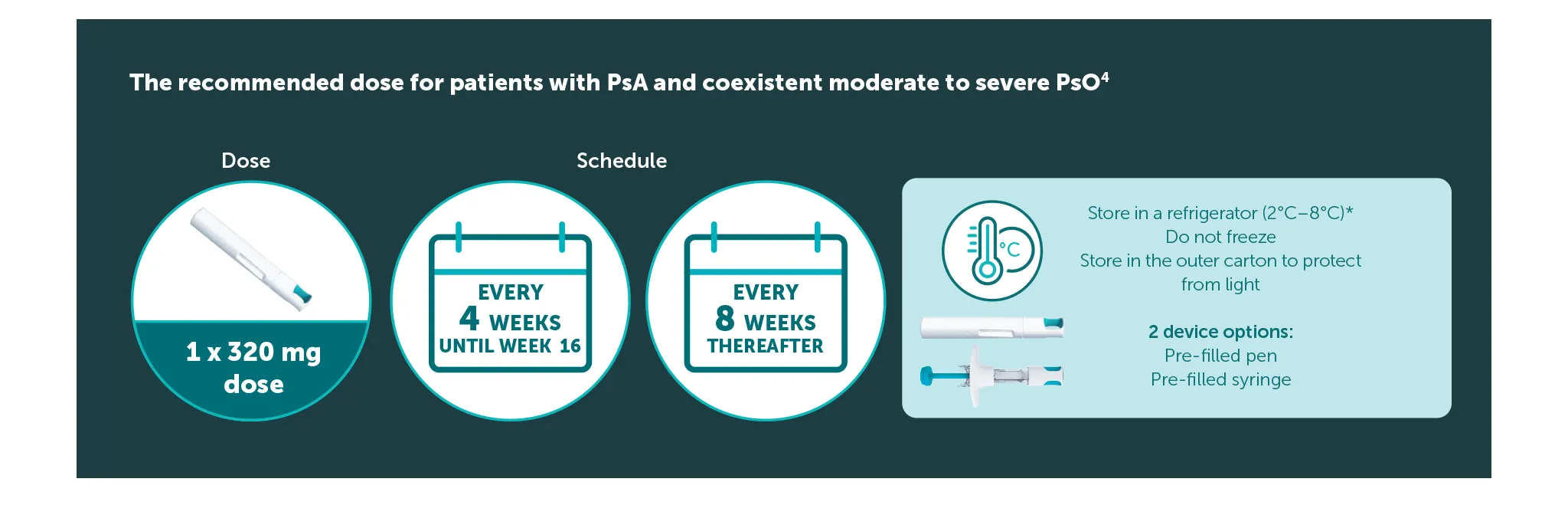
The recommended dose for adult patients with PsA and coexistent moderate to severe PsO is 320 mg (given as two subcutaneous injections of 160 mg or one subcutaneous injection of 320 mg) at Weeks 0, 4, 8, 12, 16, and every 8 weeks thereafter.4 After 16 weeks, regular assessment of efficacy is recommended and if sufficient clinical response in joints cannot be maintained, a switch to 160 mg every 4 weeks can be considered. 4 Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment. 4 For patients with a body weight ≥120 kg who do not achieved complete skin clearance at Week 16, 320 mg every 4 weeks after Week 16 may further improve treatment response.4
*The pre-filled syringe or pen may be stored at room temperature (up to 25 °C) for up to 25 days. Once removed from the refrigerator and stored under these conditions, discard after 25 days or by the expiry date printed on the container, whichever occurs first. A field for the date is provided on the carton to record the date removed from the refrigerator.4

IE-BK-2400114
Date of creation: October 2025