

▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk for the UK and hpra.ie/homepage/about-us/report-an-issue for Republic of Ireland. Adverse events should also be reported to UCB Pharma Ltd at ucbcares.uk@ucb.com or 0800 2793177 for the UK and UCB (Pharma) Ireland Ltd at ucbcares.ie@ucb.com or 1800 930075 for Republic of Ireland.
BIMZELX: THE FIRST AND ONLY APPROVED DUAL SELECTIVE INHIBITOR OF IL-17A AND IL-17F FOR USE IN PsA AND axSpA1
BIMZELX® (bimekizumab) is indicated for the treatment of: active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response or who have been intolerant to one or more DMARDs; active nr-axSpA, in adults with objective signs of inflammation as indicated by elevated CRP and/or MRI, who have responded inadequately or are intolerant to NSAIDs; and active AS, in adults who have responded inadequately or are intolerant to conventional therapy.1
IL-17 plays an important role in psoriasis, PsA and axSpA with levels of IL-17A and IL-17F often elevated in patients with PsA and axSpA2,3
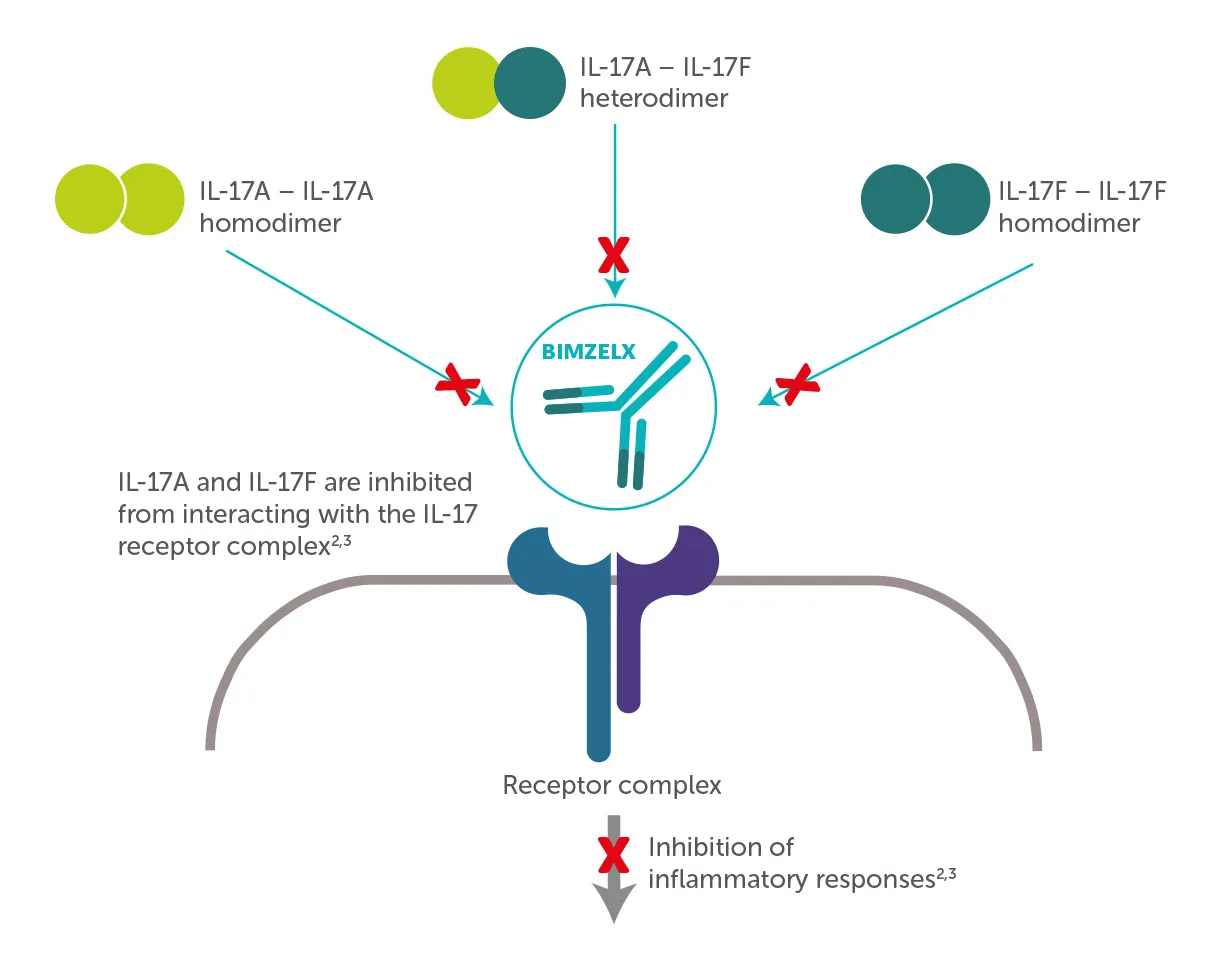
The clinical significance of this is yet to be determined
Adapted from, Reich, K,. et al. Lancet 2021
IE-BK-2300067
Date of creation: October 2025
