▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk for the UK and hpra.ie/homepage/about-us/report-an-issue for Republic of Ireland. Adverse events should also be reported to UCB Pharma Ltd at ucbcares.uk@ucb.com or 0800 2793177 for the UK and UCB (Pharma) Ireland Ltd at ucbcares.ie@ucb.com or 1800 930075 for Republic of Ireland.
BIMZELX ACHIEVED A CONSISTENT RESPONSE ACROSS THE axSpA SPECTRUM WHICH WAS SUSTAINED OVER TIME1–9
BIMZELX® (bimekizumab) is indicated for the treatment of: active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response or who have been intolerant to one or more DMARDs; active nr-axSpA, in adults with objective signs of inflammation as indicated by elevated CRP and/or MRI, who have responded inadequately or are intolerant to NSAIDs; and active AS, in adults who have responded inadequately or are intolerant to conventional therapy.7
CHALLENGE LIMITATIONS IN axSpA WITH BIMZELX
EFFICACY

Explore more in ‘Symptom improvement’ section below |
Explore more in ‘Reductions in disease activity’ section below |

Explore more in ‘Inflammation control’ section below |

Explore more in ‘Inhibition of disease progression’ section below |

Explore more in ‘Resolution of enthesitis’ section below |
Explore more in ‘Patient-reported outcomes’ section below |
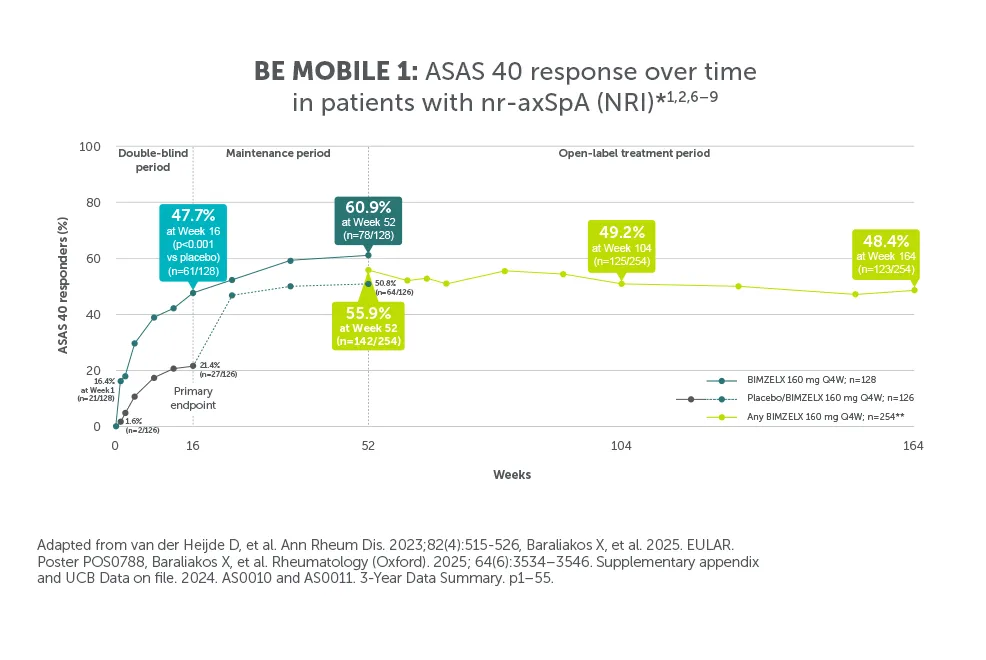
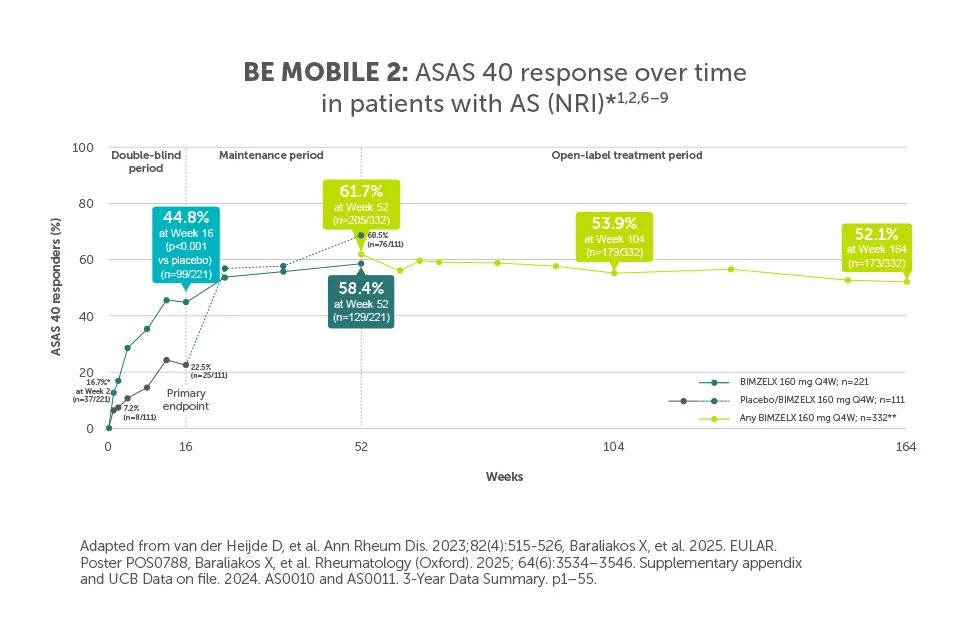
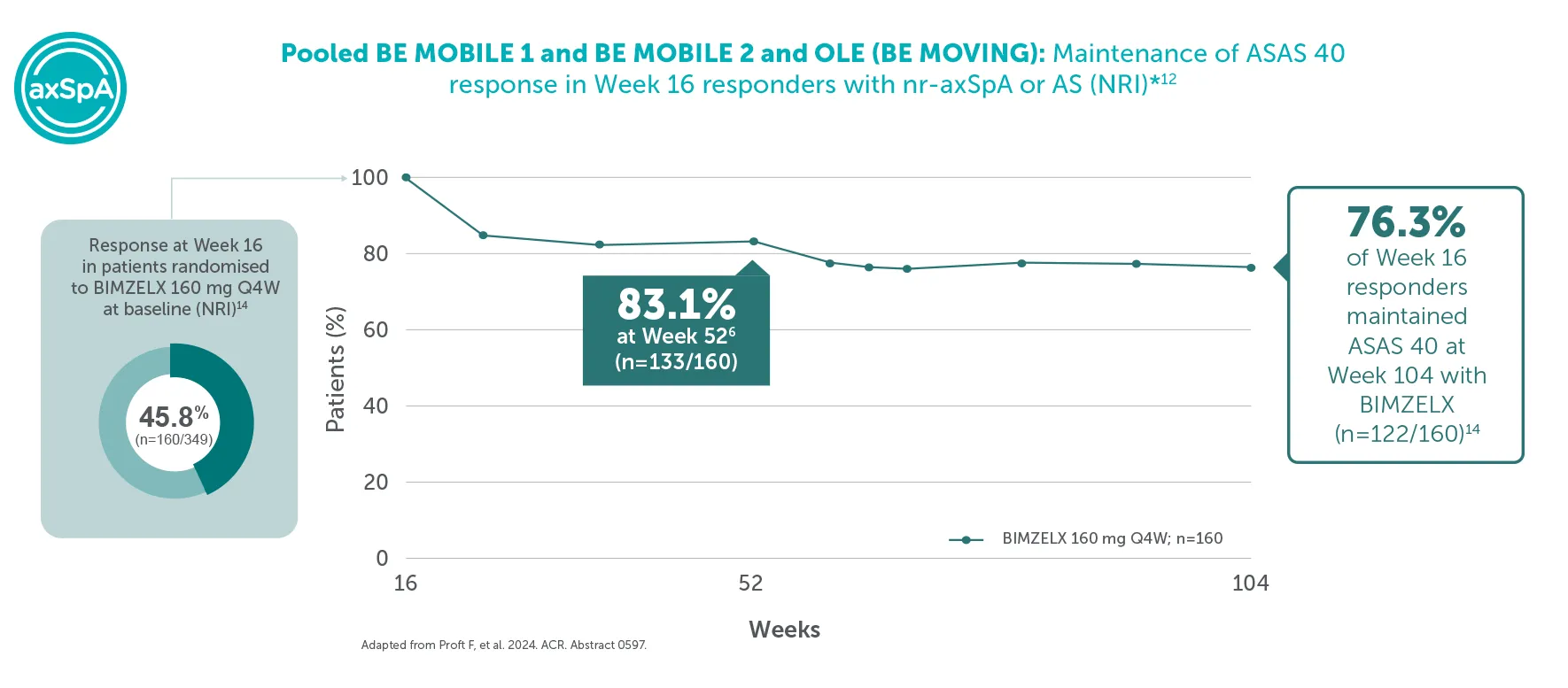
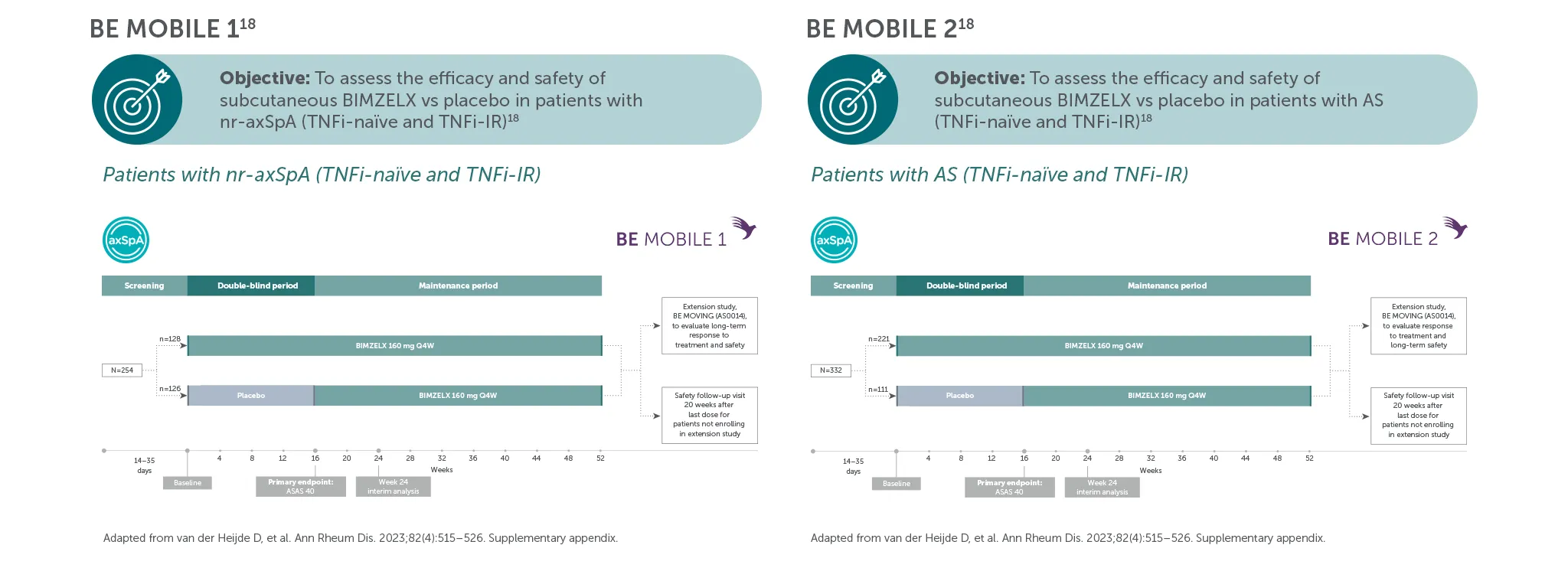
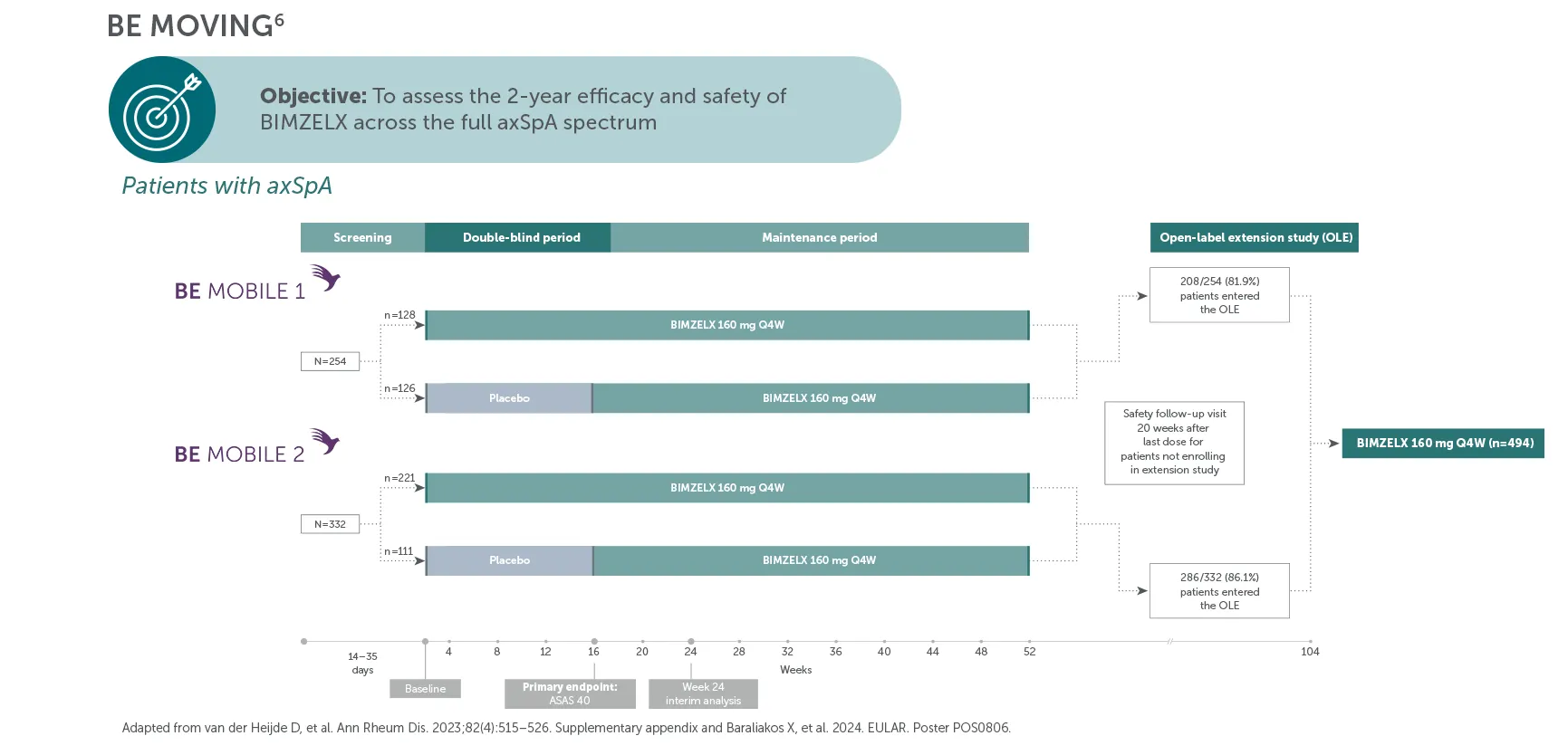
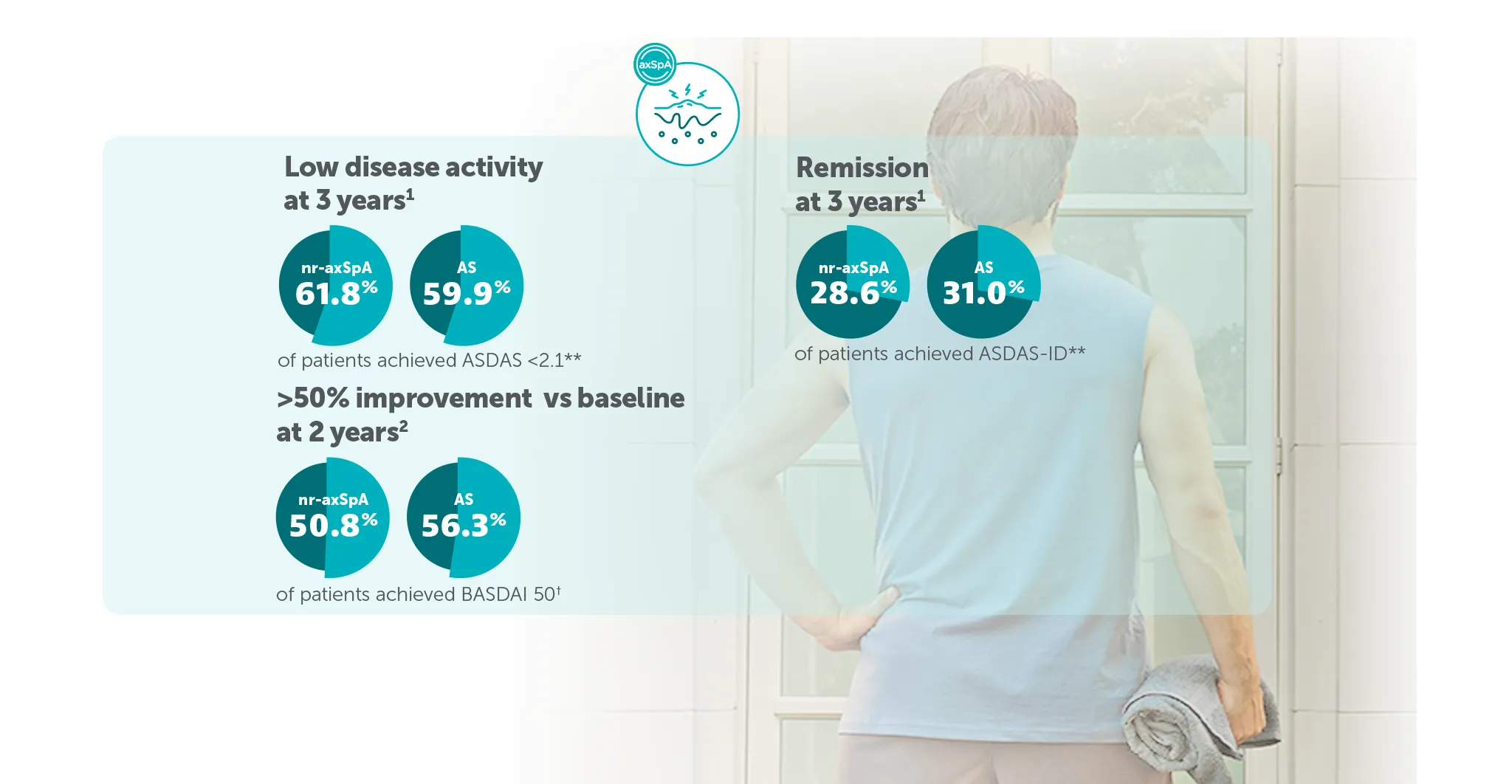
*In BE MOBILE 1 and BE MOBILE 2, ASAS 40 was achieved by 47.7% (61/128) of nr-axSpA patients and 44.8% (99/221) of AS patients at Week 16, (primary endpoint; vs 21.4% (27/126) and 22.5% (25/111) with placebo, respectively; p<0.001);6 60.9% (78/128) and 58.4% (129/221) at Week 52, respectively (NRI analysis).6,7 In the open-label treatment period, ASAS 40 was achieved by 50.8% (129/254) of nr-axSpA patients and 55.1% (183/332) of AS patients receiving BIMZELX at Week 104; 48.4% (123/254) and 52.1% (173/332) at Week 164, respectively (NRI analysis).1,8
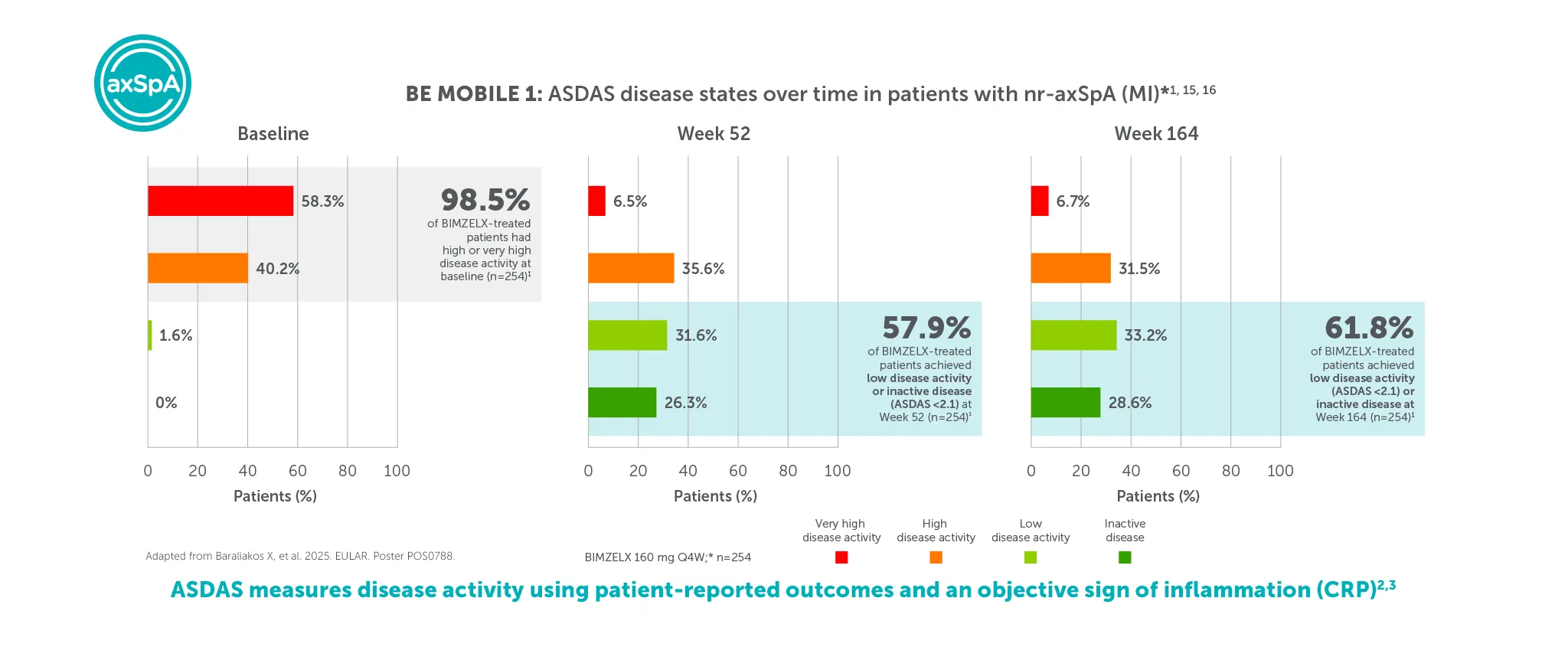
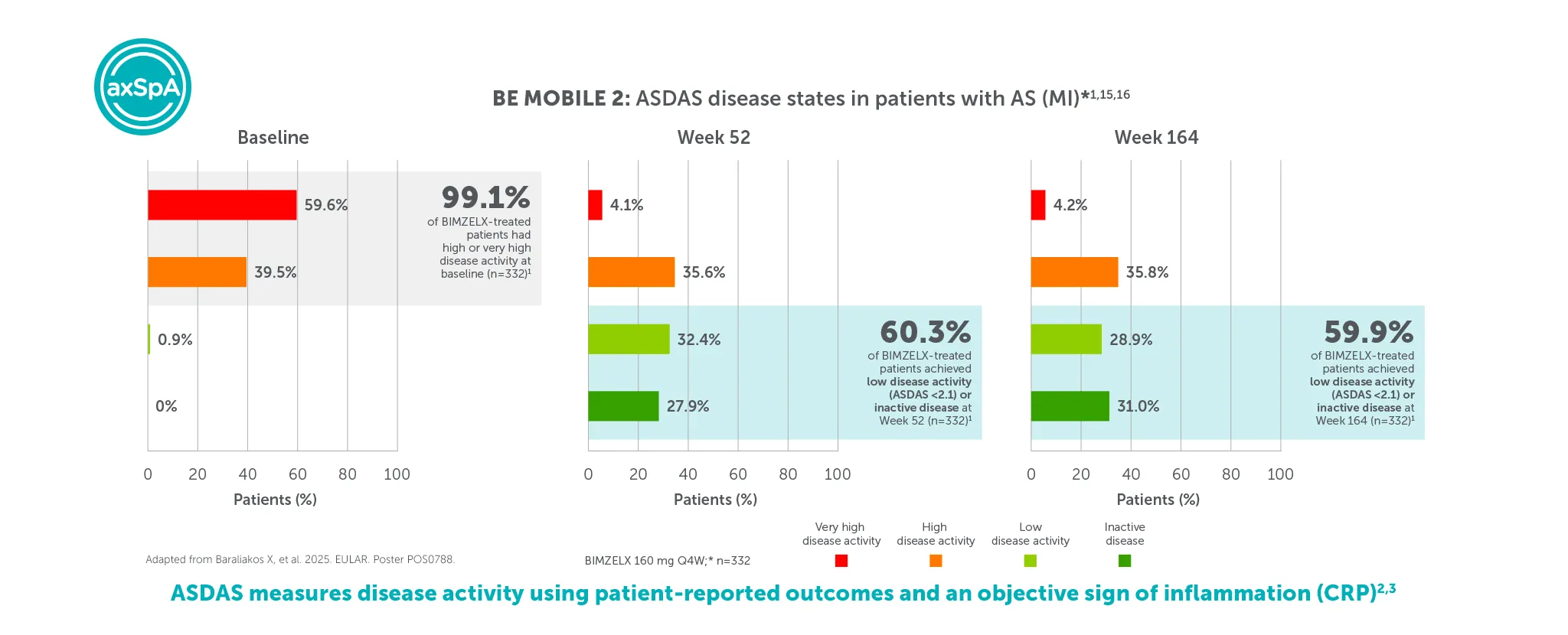
**In BE MOBILE 1 and the BE MOVING OLE, ASDAS <2.1 was achieved by 46.1% (59/128) at Week 16, 61.6% (79/128) at Week 52,9 61.4% (n=254) at Week 104 and 61.8% (n=254) at Week 164, of patients with nr-axSpA in the BIMZELX treatment arm (pooled randomised set, secondary endpoint, MI analysis).1 In BE MOBILE 2 and the BE MOVING OLE, ASDAS <2.1 was achieved by 44.8% (99/221) at Week 16, 57.1% (126/221) at Week 52,9 63.3% (n=332) at Week 104 and 59.9% (n=332) of patients with AS at Week 164 (pooled randomised set, secondary endpoint, MI analysis).1 In BE MOBILE 1/2 and the BE MOVING OLE, ASDAS-ID was achieved by 28.6% (n=254) of nr-axSpA patients and 31.0% (n=332) of AS patients at Week 164, respectively (MI analysis).1
†BASDAI 50 was achieved by 50.8% (129/254) of nr-axSpA patients and 56.3% (187/332) of AS patients receiving BIMZELX at Week 104 in the BE MOVING OLE (pre-specified endpoint in BE MOVING; NRI analysis).2
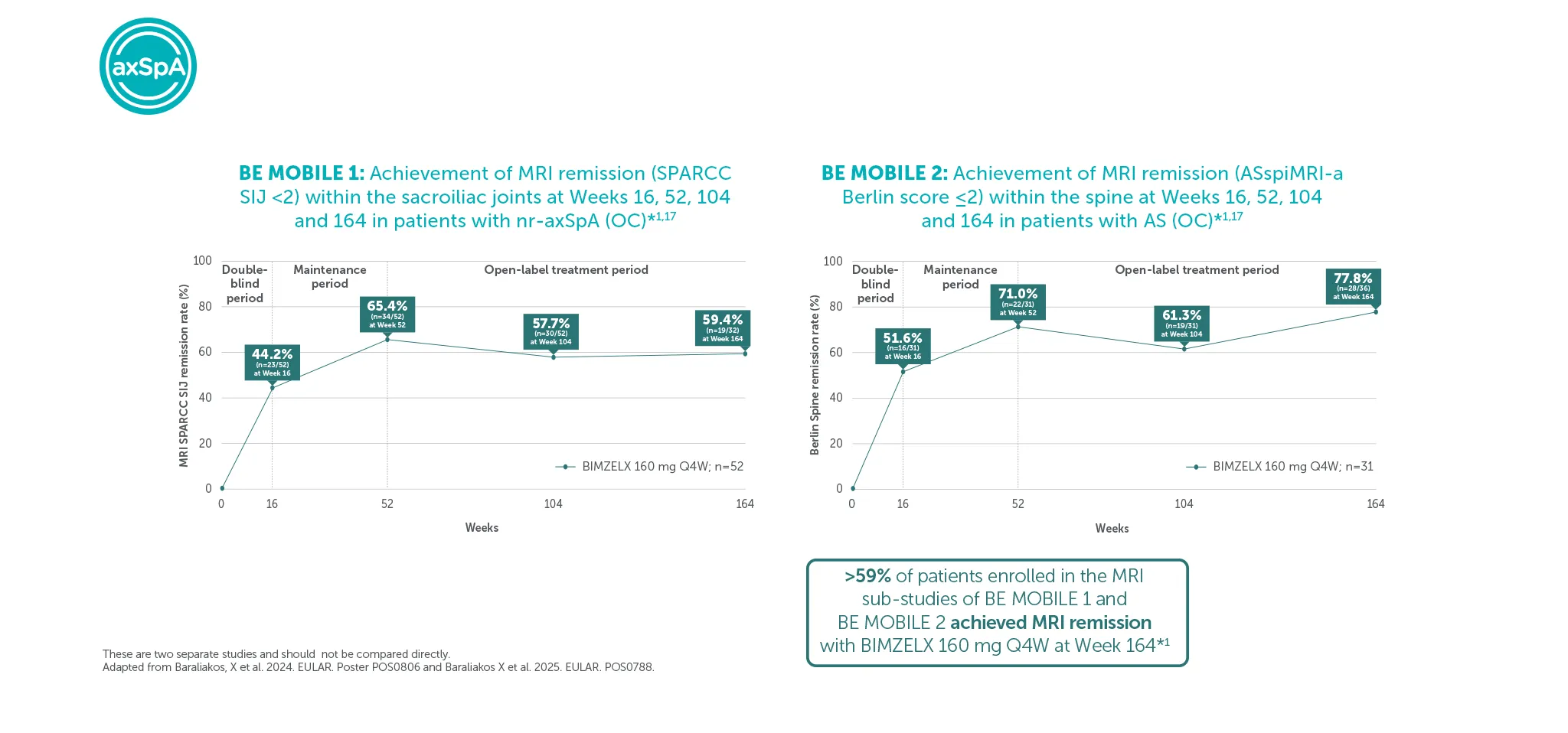
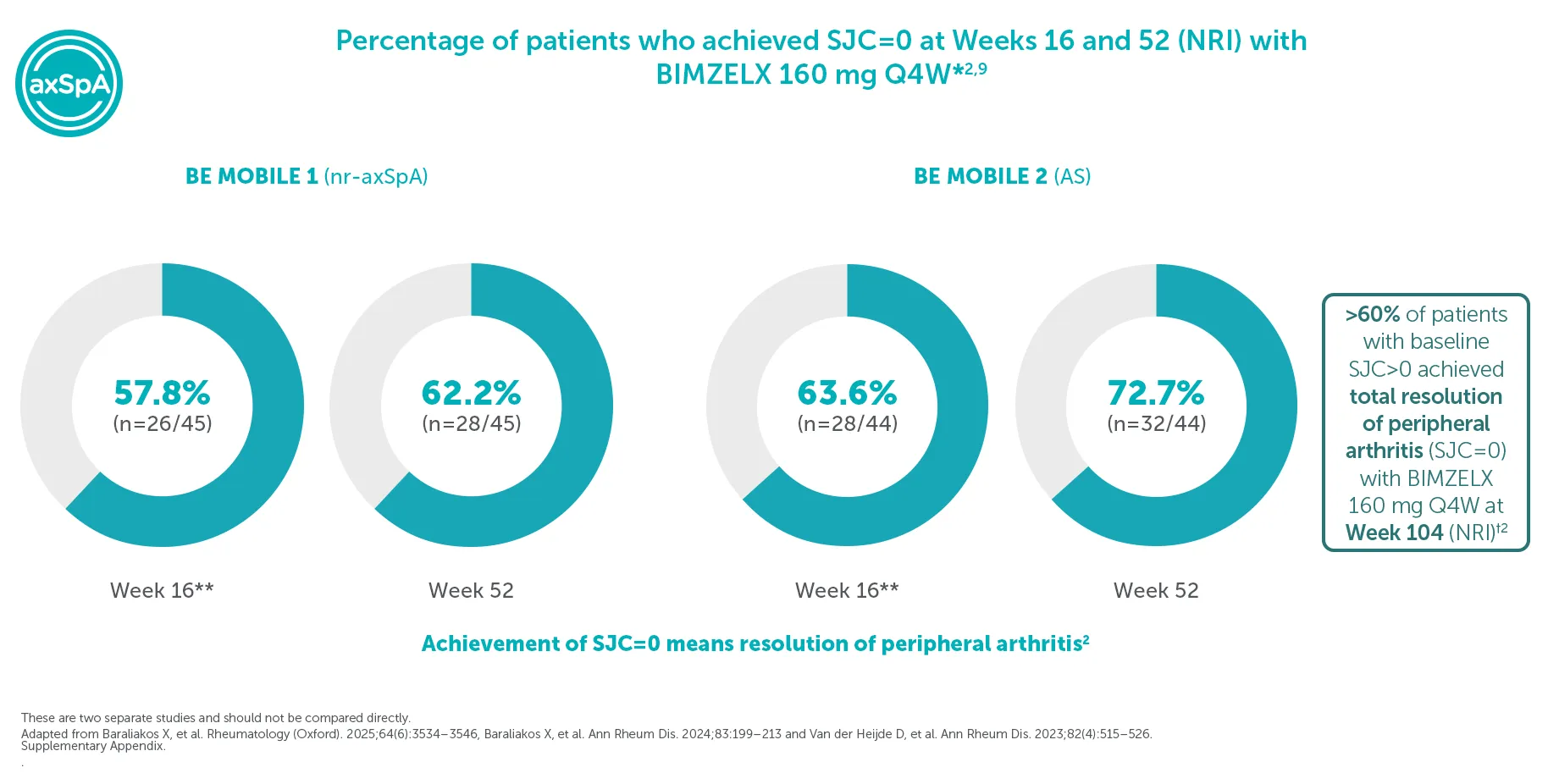
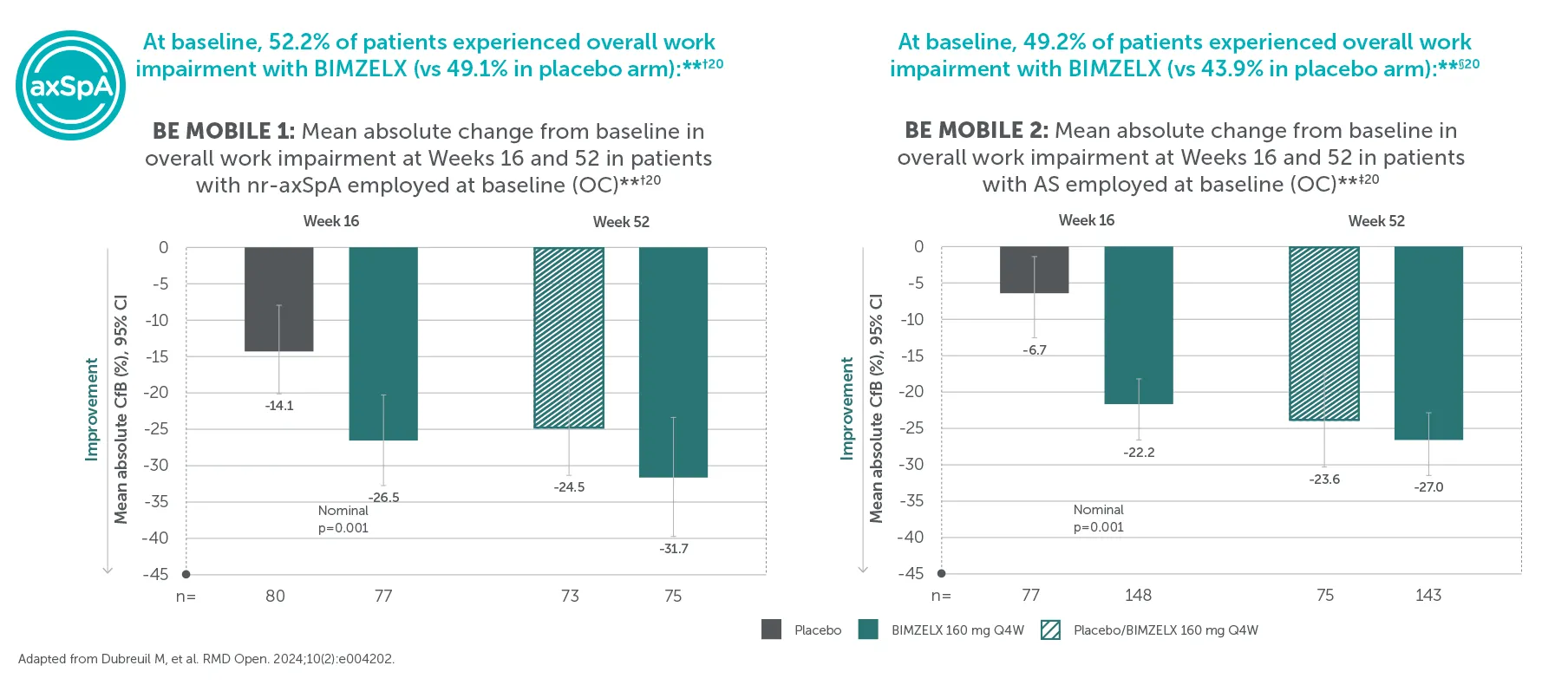
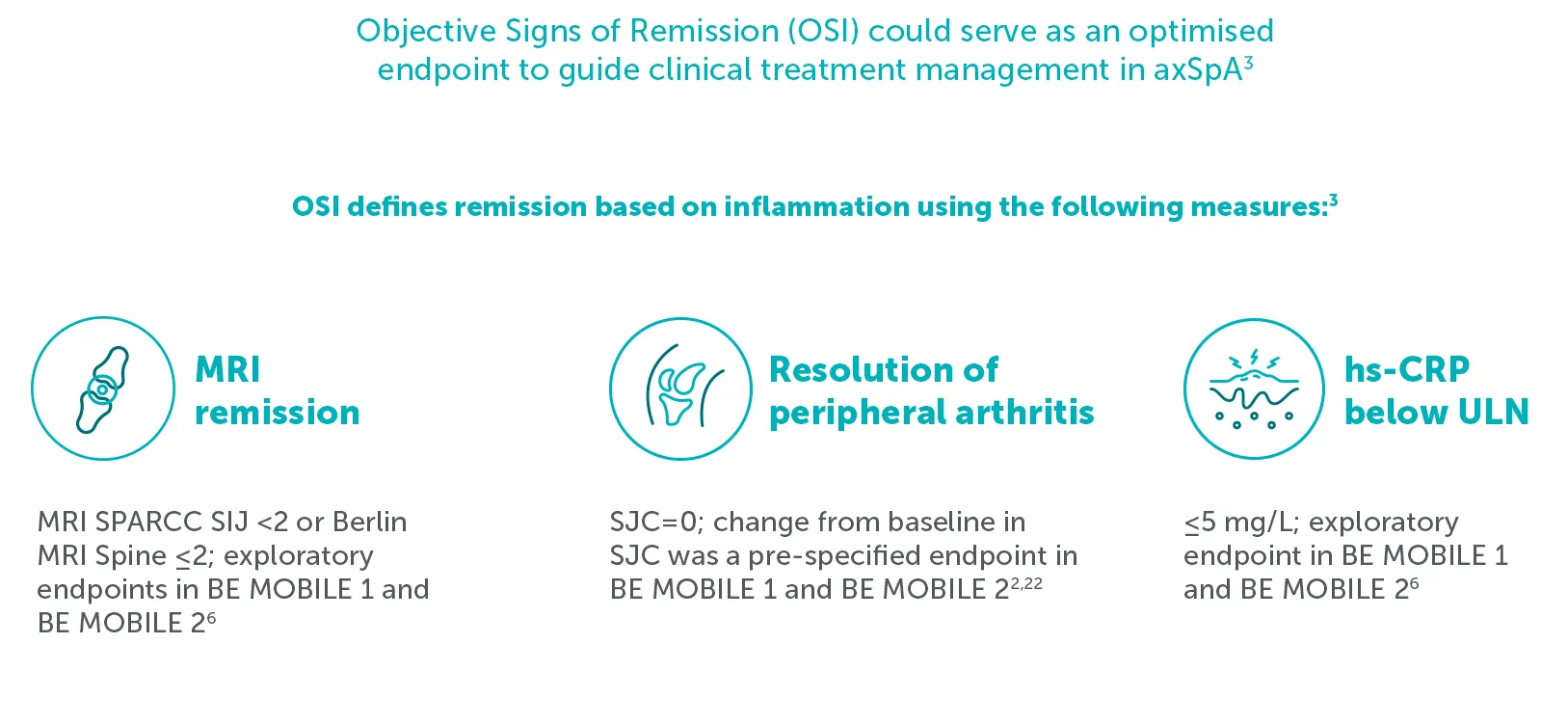
‡In BE MOBILE 1 and BE MOBILE 2, at Week 52, 50.8% (32/63) of nr-axSpA patients and 50.0% (38/76) AS patients in the BIMZELX treatment arm achieved remission defined by absence of OSI (MRI SPARCC SIJ <2; Berlin MRI Spine ≤2; SJC=0; hs-CRP (mg/L) ≤5). 152 and 139 patients from the MRI sub-studies of BE MOBILE 1 and BE MOBILE 2, respectively, were included in the post-hoc analysis. Levels of objective signs of inflammation at baseline were similar across treatment arms.3
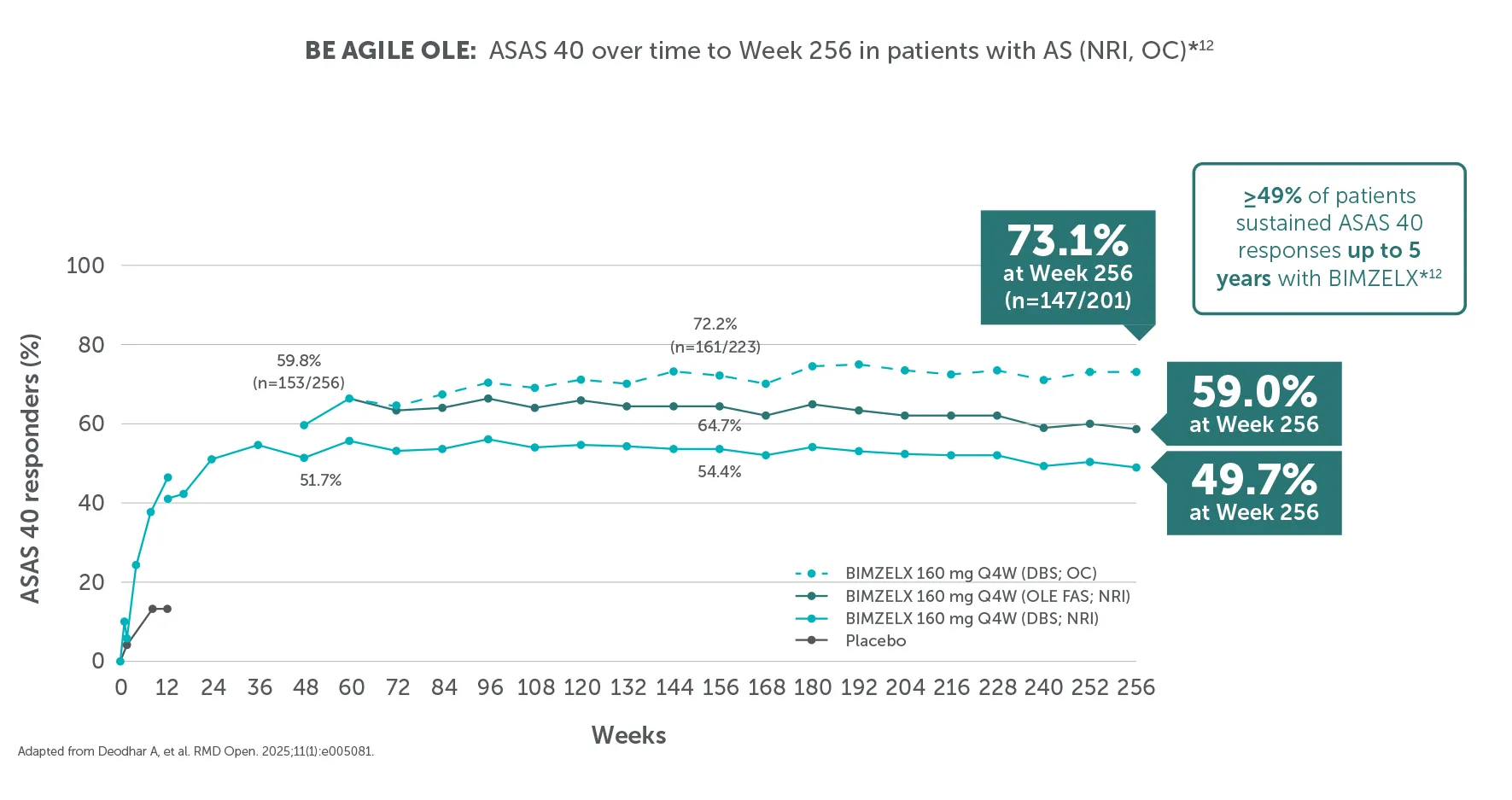
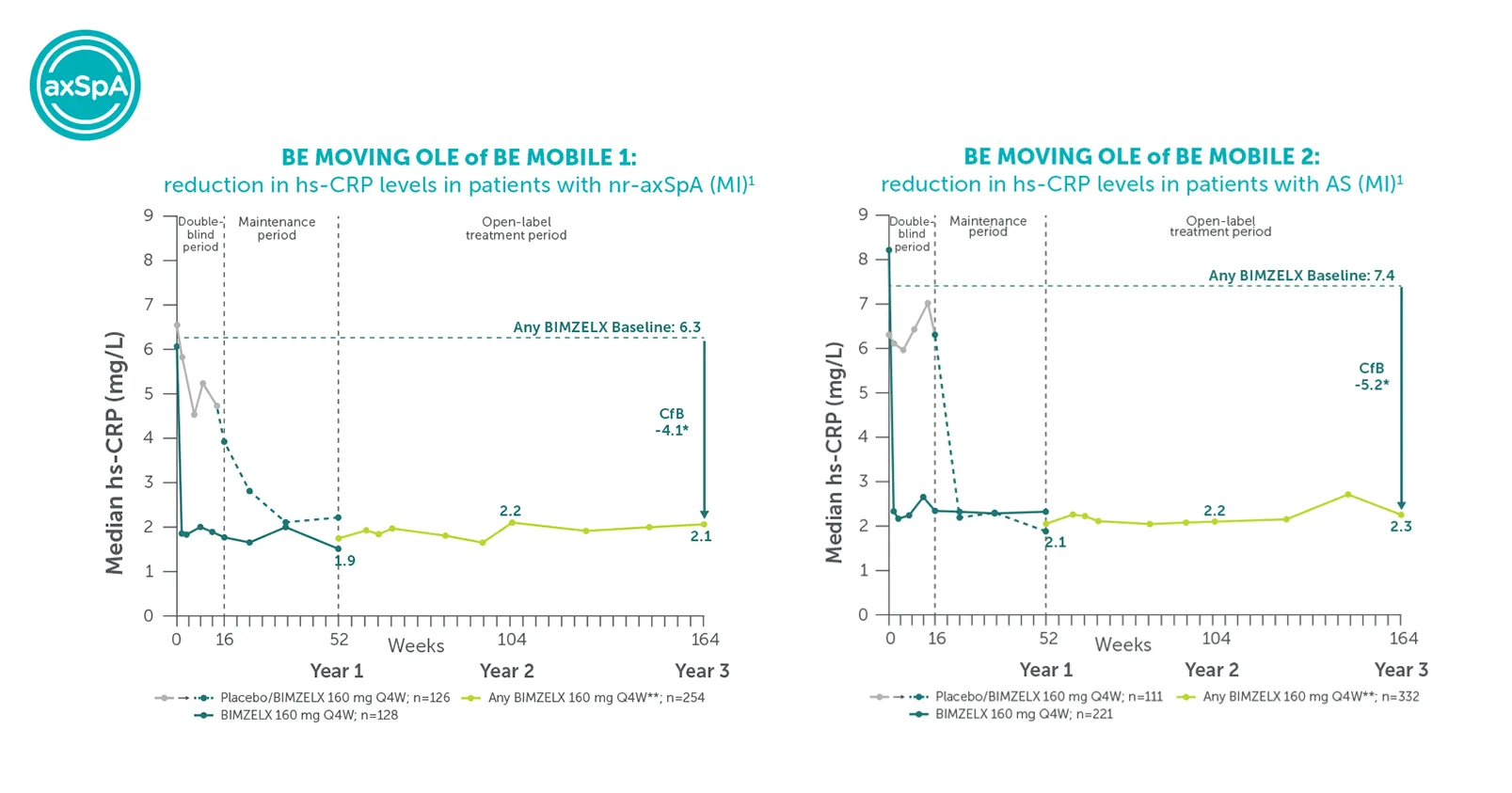
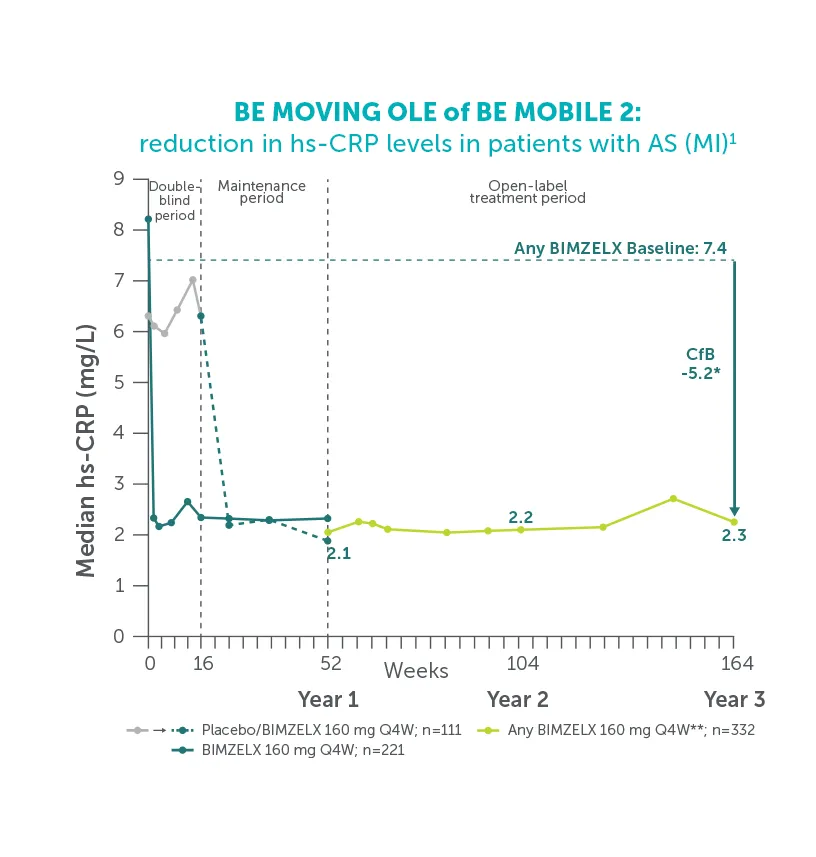
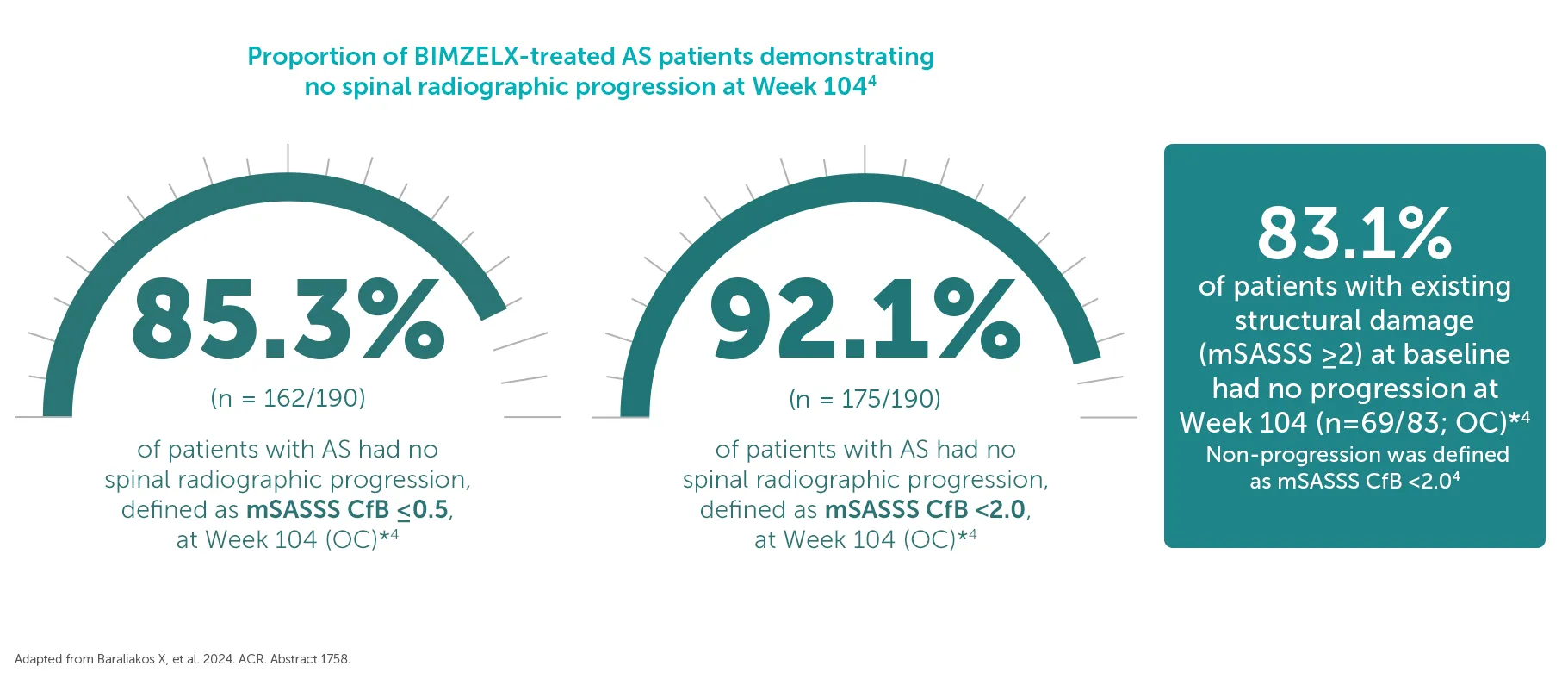
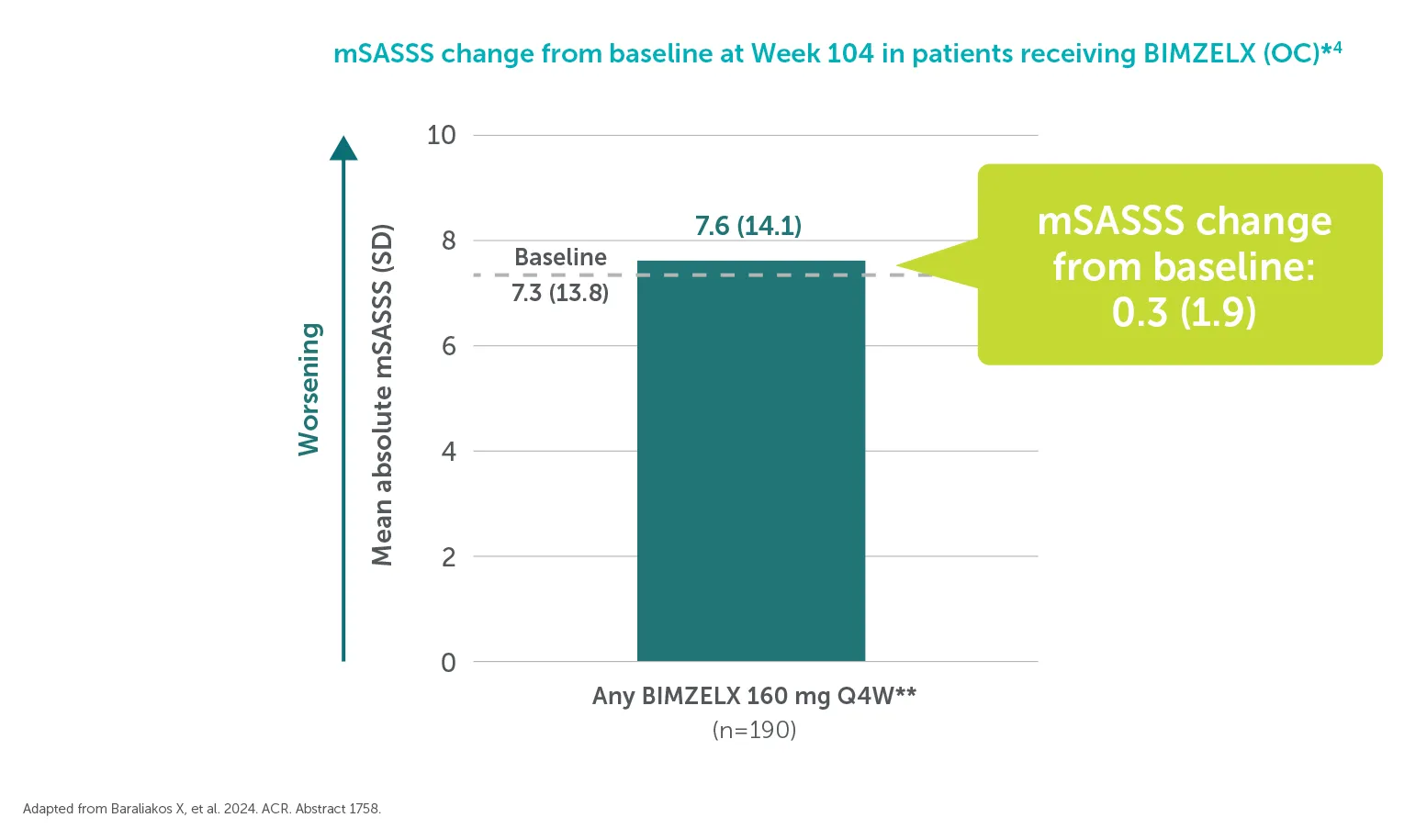
§In the BE MOVING OLE of BE MOBILE 2, 85.3% (162/190) of AS patients had no spinal radiographic progression (mSASSS ≤0.5) at Week 104 (OC analysis).4
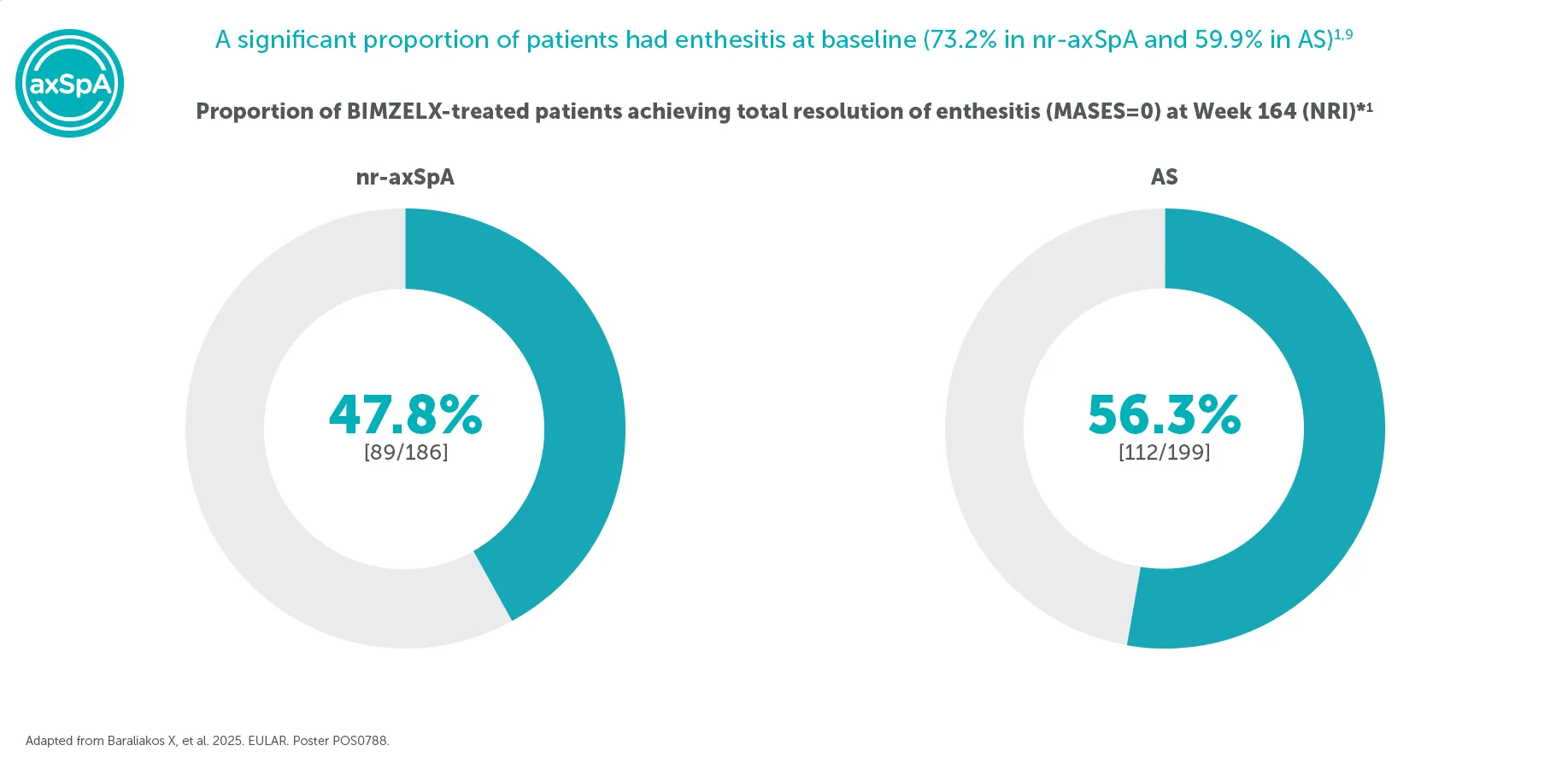
¥In the BE MOVING OLE of BE MOBILE 1 and BE MOBILE 2, complete resolution of enthesitis (exploratory endpoint, MASES=0; NRI analysis) at Week 164 was achieved by 47.8% (n=186) of BIMZELX-treated patients with nr-axSpA and 56.3% (n=199) of patients with AS, respectively.1
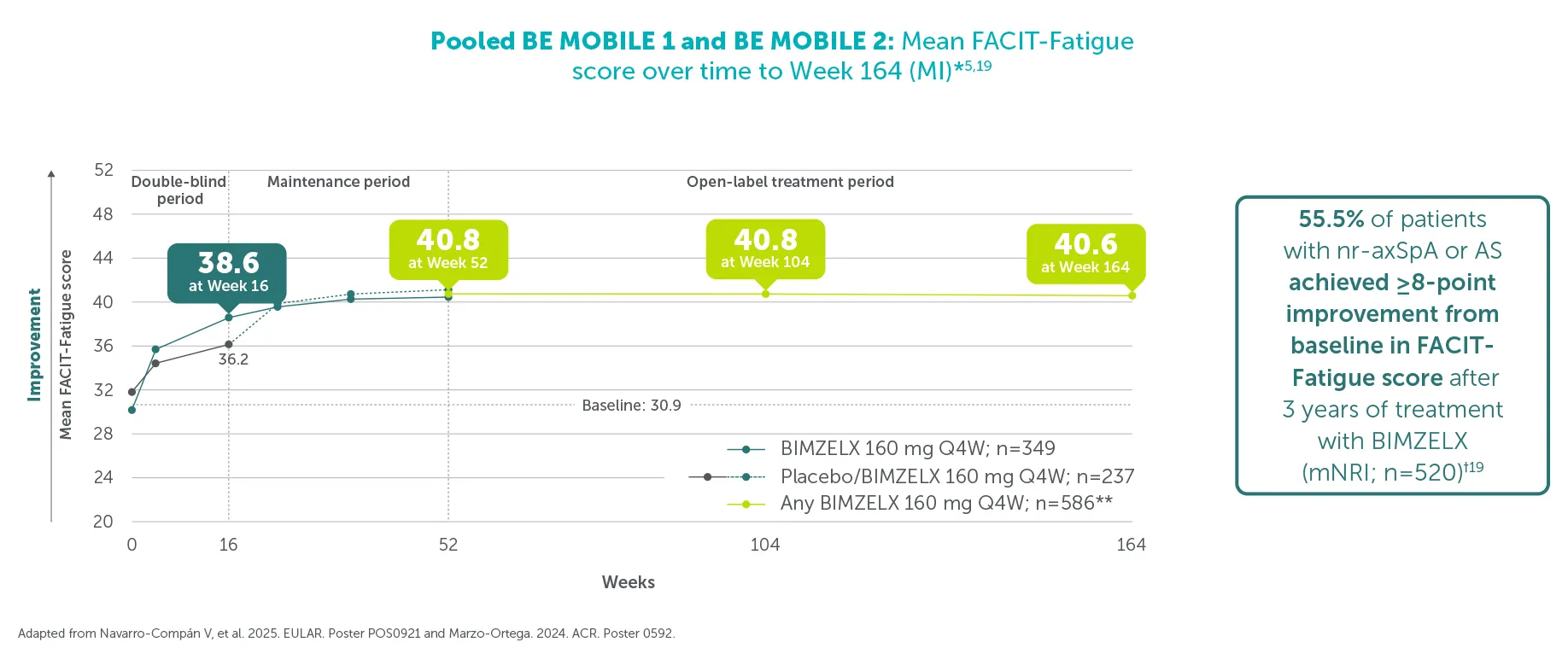
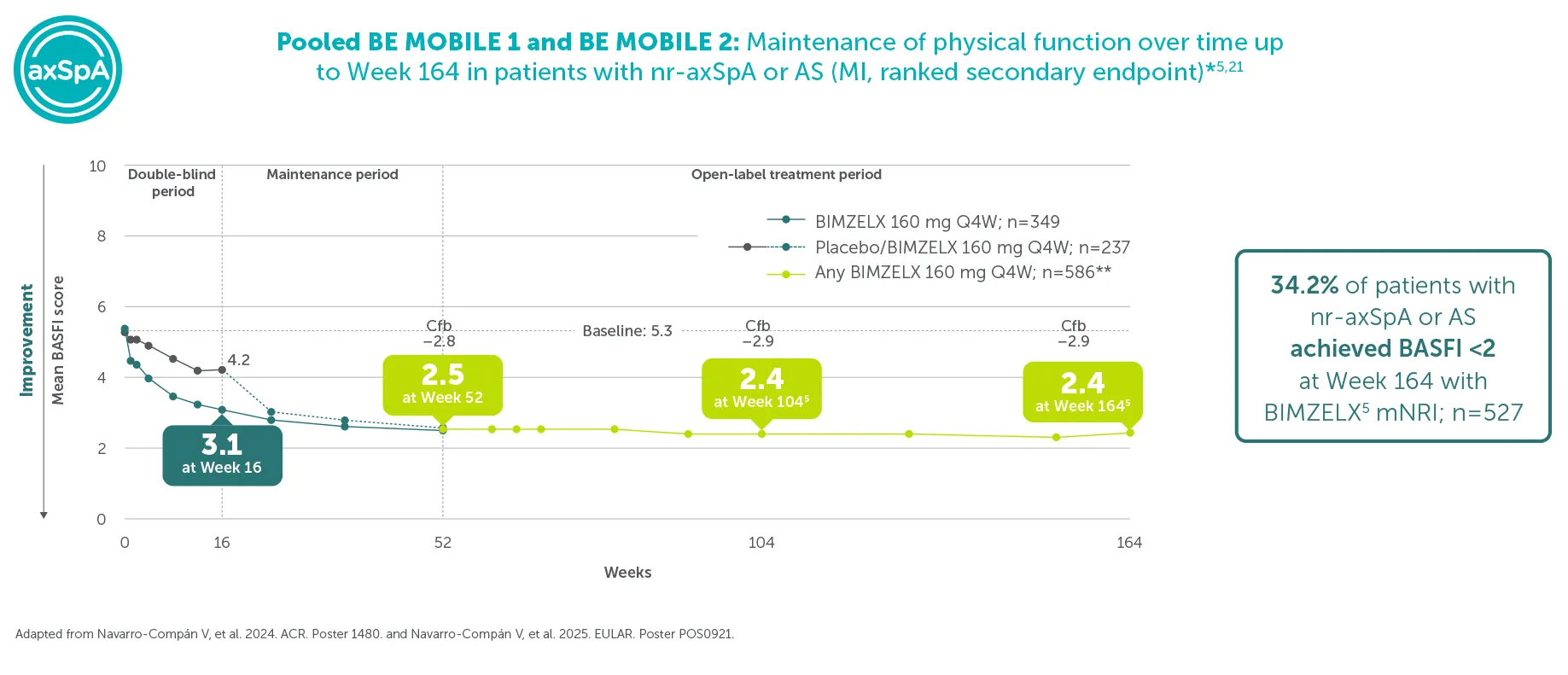
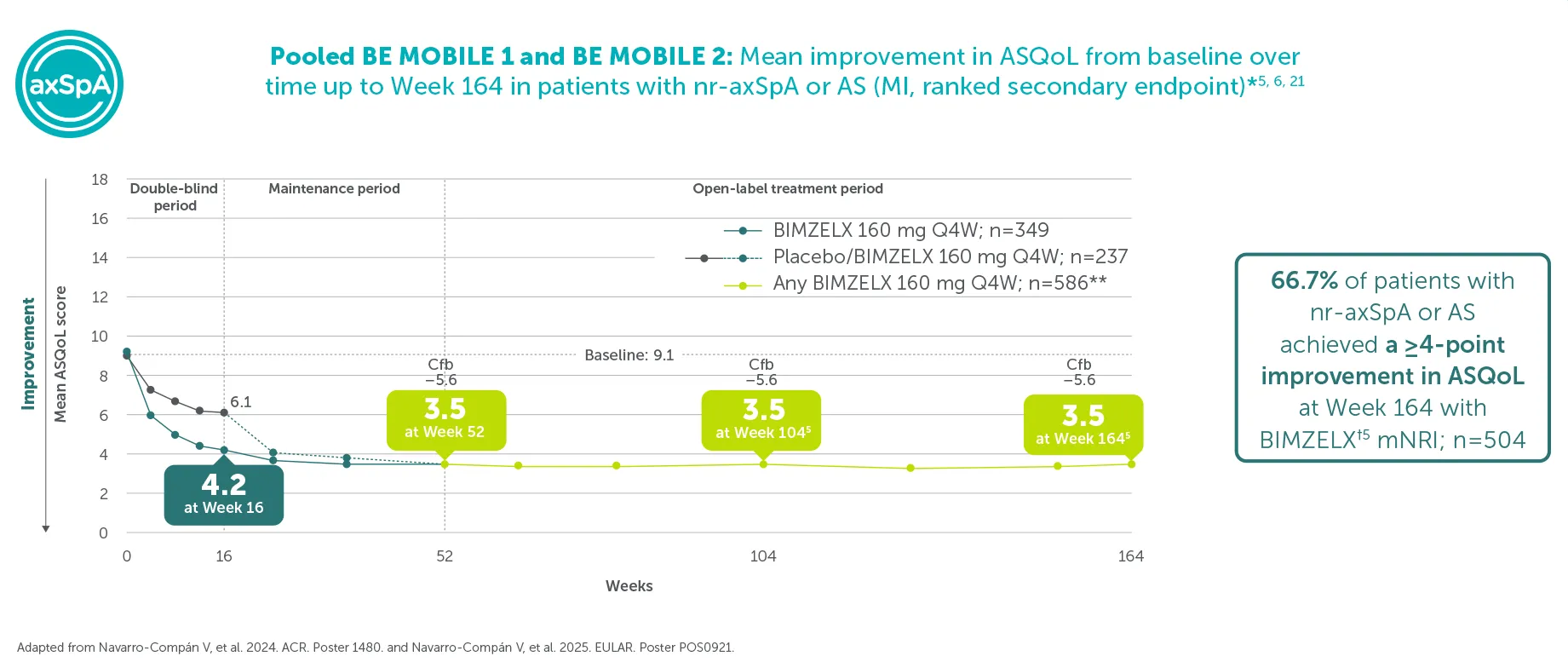
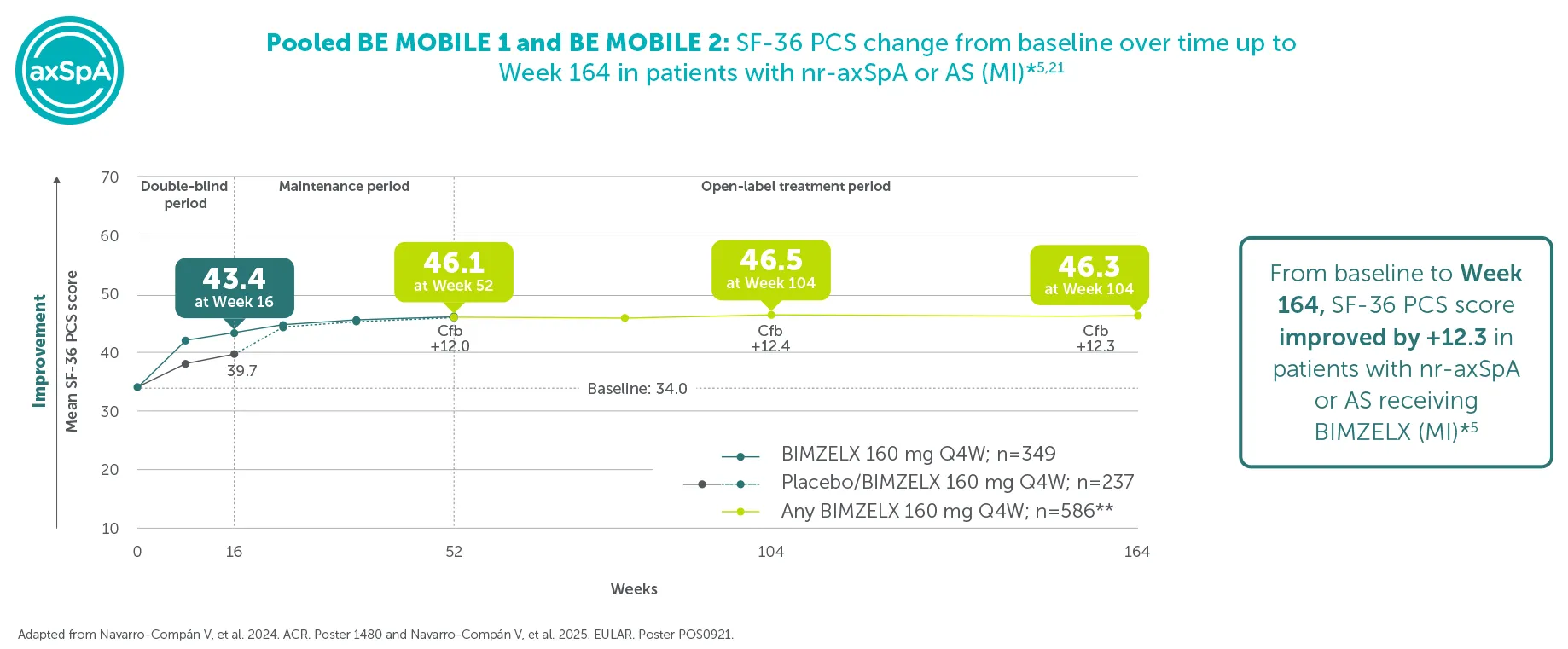
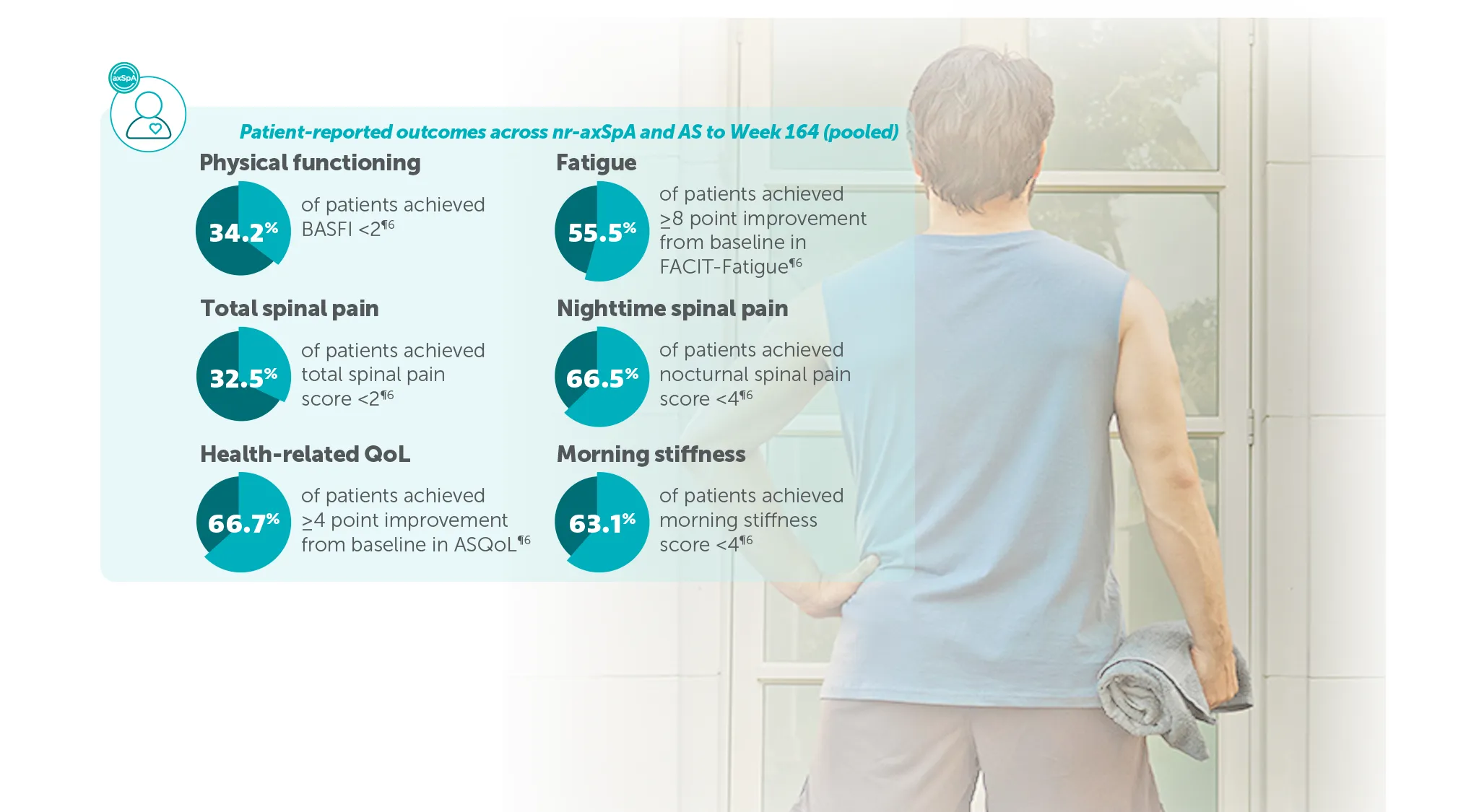
¶In pooled analyses of patients with nr-axSpA or AS treated with BIMZELX 160 mg Q4W in BE MOBILE 1 and BE MOBILE 2, respectively, up to 3 years (populations include patients originally randomised to placebo):
34.2% (n=586) achieved BASFI <2 (mNRI)5
66.7% (n=504) achieved ≥4-point improvement in ASQoL (in patients with ASQoL score ≥4 at baseline; mNRI)5
55.5% (n=520) achieved ≥8-point improvement from baseline in FACIT-Fatigue score (in patients with FACIT-Fatigue score ≤44 at baseline)5
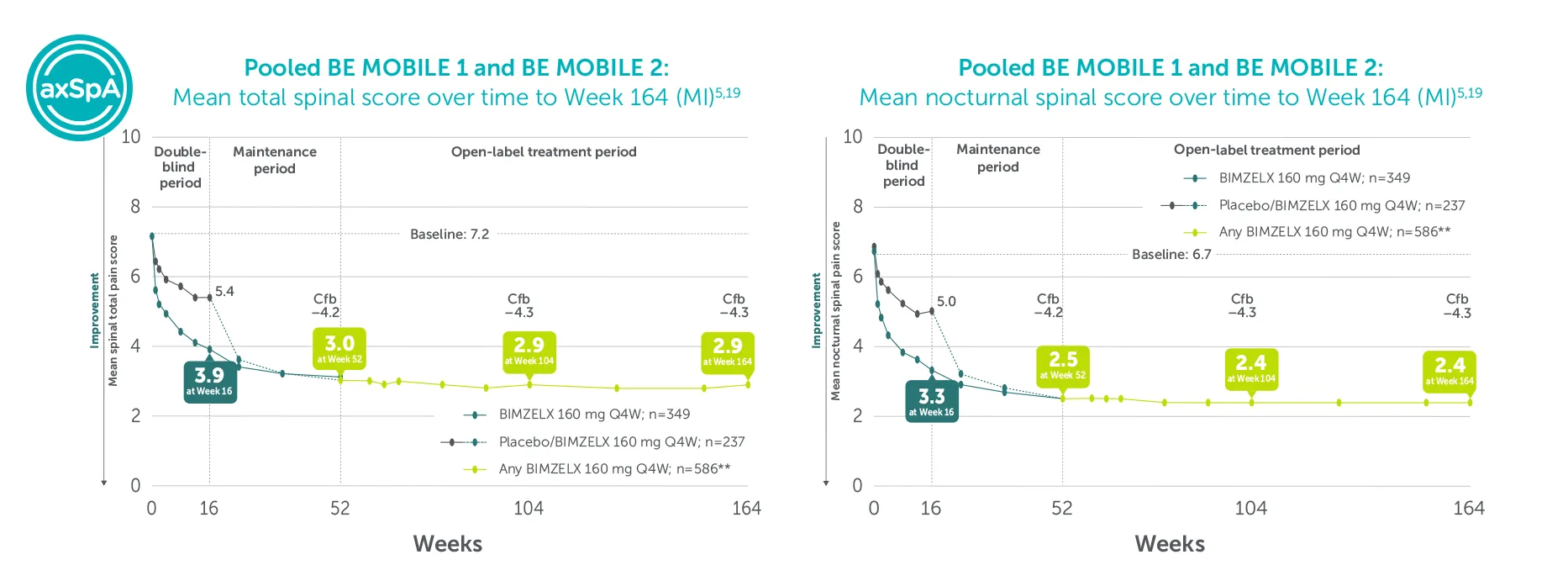
66.5% (n=586) achieved nocturnal spinal pain score <4 (mNRI)5
32.5% (n=586) achieved total spinal pain score <2 (mNRI)5
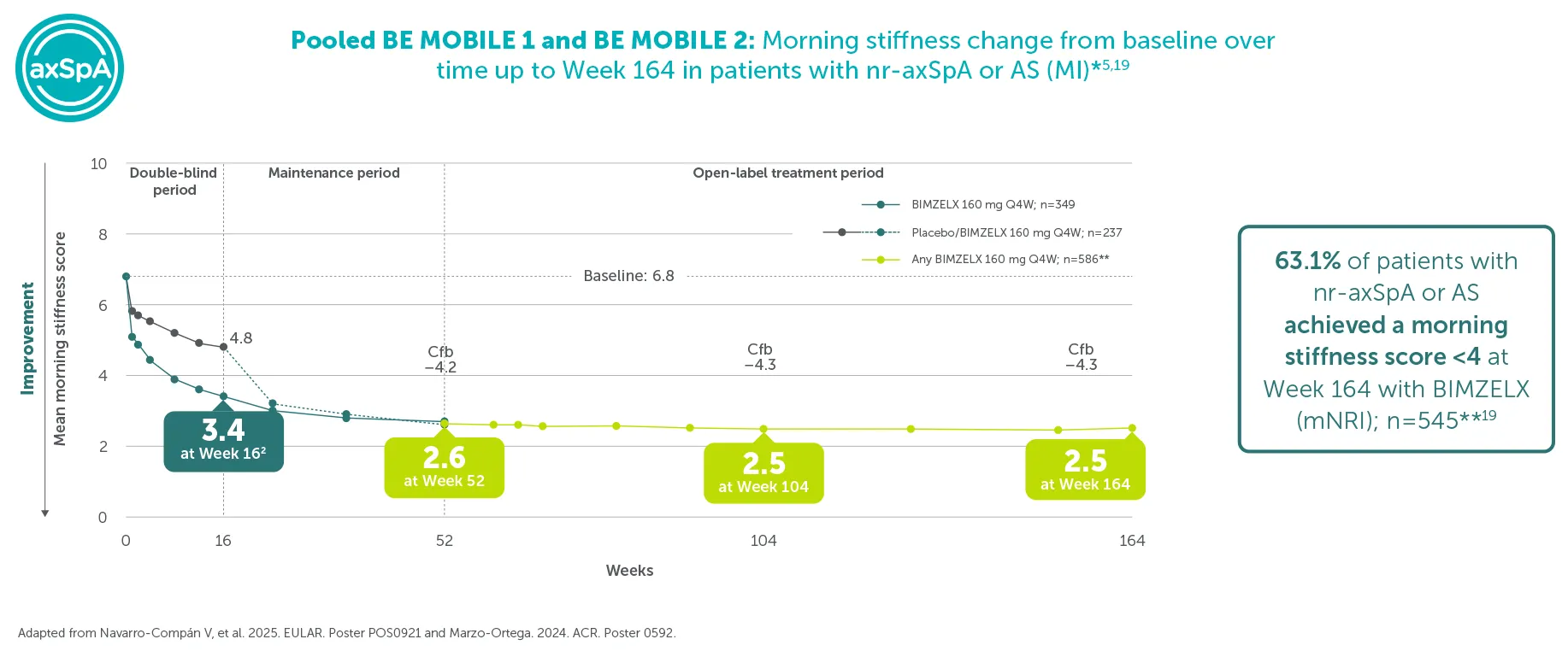
63.1% (n=586) achieved morning stiffness score <4 (mean of BASDAI Q5 and 6; mNRI)5
Improvements in BASDAI, a key measure of disease activity, were sustained to Week 104 in BIMZELX-treated patients2,22
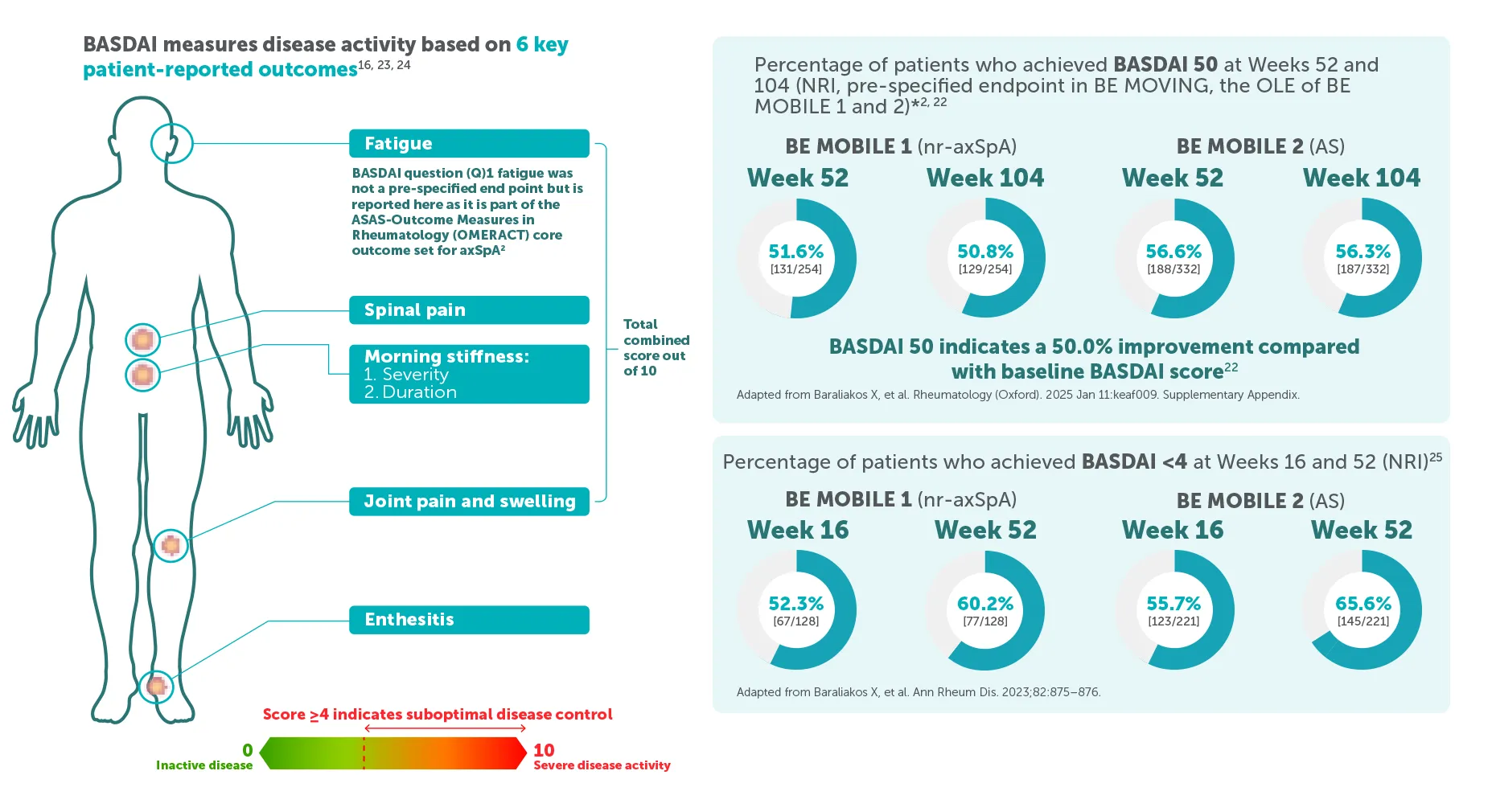
*BE MOBILE 1 baseline mean BASDAI scores: 6.9 in the BIMZELX treatment group vs 6.7 in the placebo group.22 BE MOBILE 2 baseline mean BASDAI scores: 6.5 in the BIMZELX treatment group vs 6.5 in the placebo group.22 Includes patients who were originally assigned to placebo.2 Disease activity (BASDAI) was a ranked secondary endpoint in BE MOBILE 1 and 2 and a pre-specified endpoint in BE MOVING.2,22
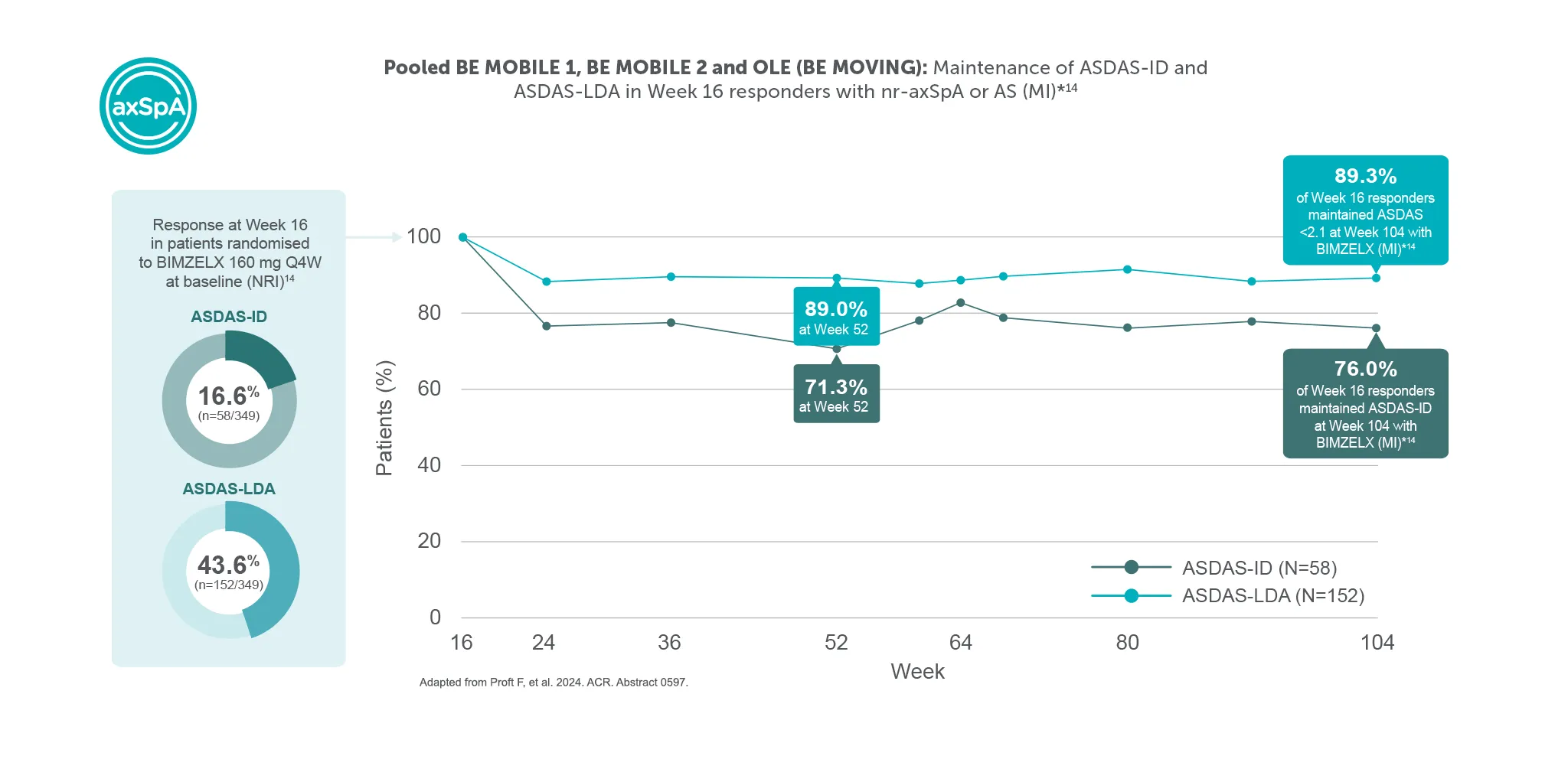
ASDAS-ID, an established measure of remission, may underestimate the anti-inflammatory effects of BIMZELX3
SAFETY
BIMZELX was generally well tolerated, with long-term exposure up to 3 years13,32
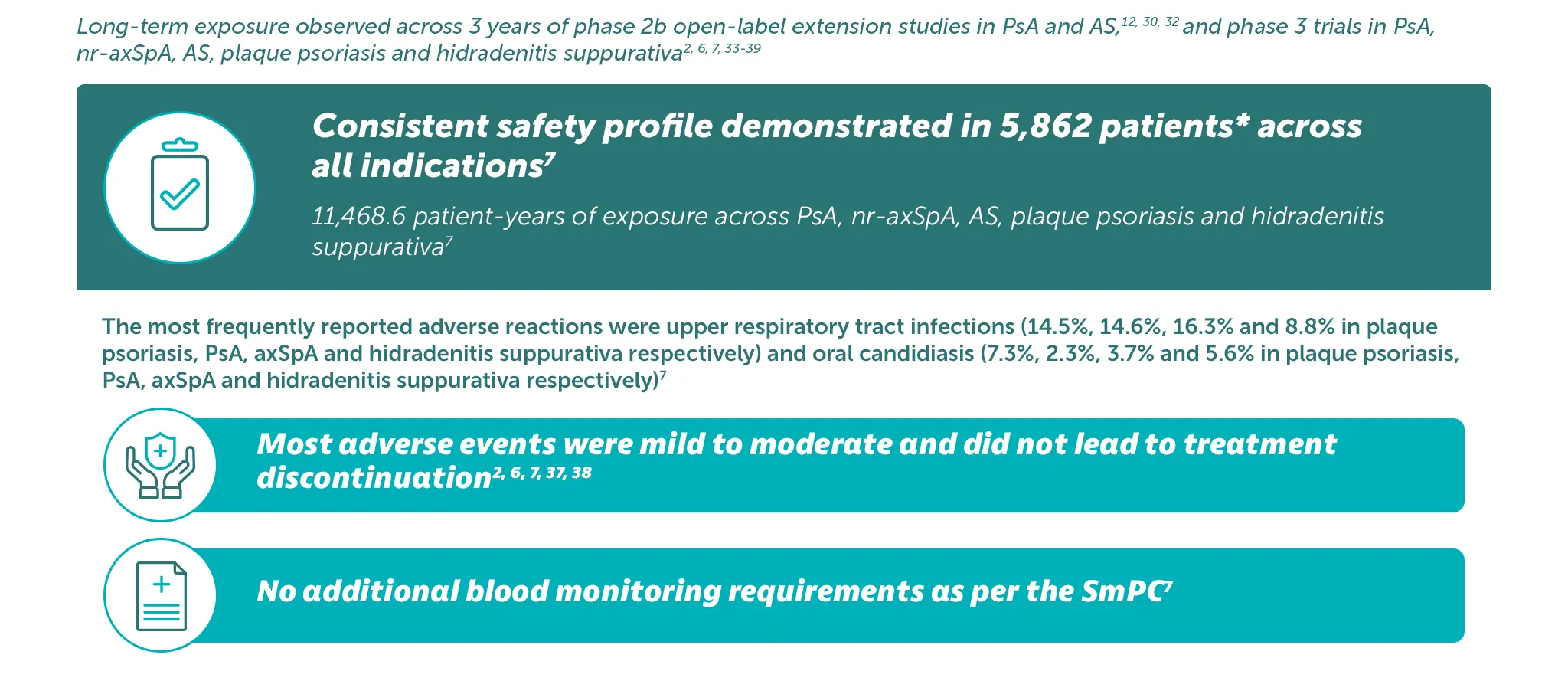
Adverse events: Refer to SmPC for full information. Very common (≥1/10): Upper respiratory tract infection; Common (≥1/100 to< 1/10): Oral candidiasis, tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, dermatitis and eczema, acne, injection site reactions, fatigue; Uncommon (≥1/1,000 to<1/100): Mucosal and cutaneous candidiasis (including oesophageal candidiasis), conjunctivitis, neutropenia, inflammatory bowel disease. *5,862 patients treated in blinded and open-label clinical studies in PsA, nr-axSpA, AS, moderate to severe plaque psoriasis, and moderate-to-severe HS. Of these, over 4,660 patients were exposed to BIMZELX for at least one year.4
BIMZELX has a consistent long-term safety profile in patients with axSpA and PsA26
BIMZELX is generally well tolerated in patients with axSpA and PsA as seen by long-term exposure analyses from phase 2b/3 trials in patients with at least 104 weeks of total study participation26
Pooled analysis of patients with axSpA (nr-axSpA or AS): Overview of TEAEs up to data-cut
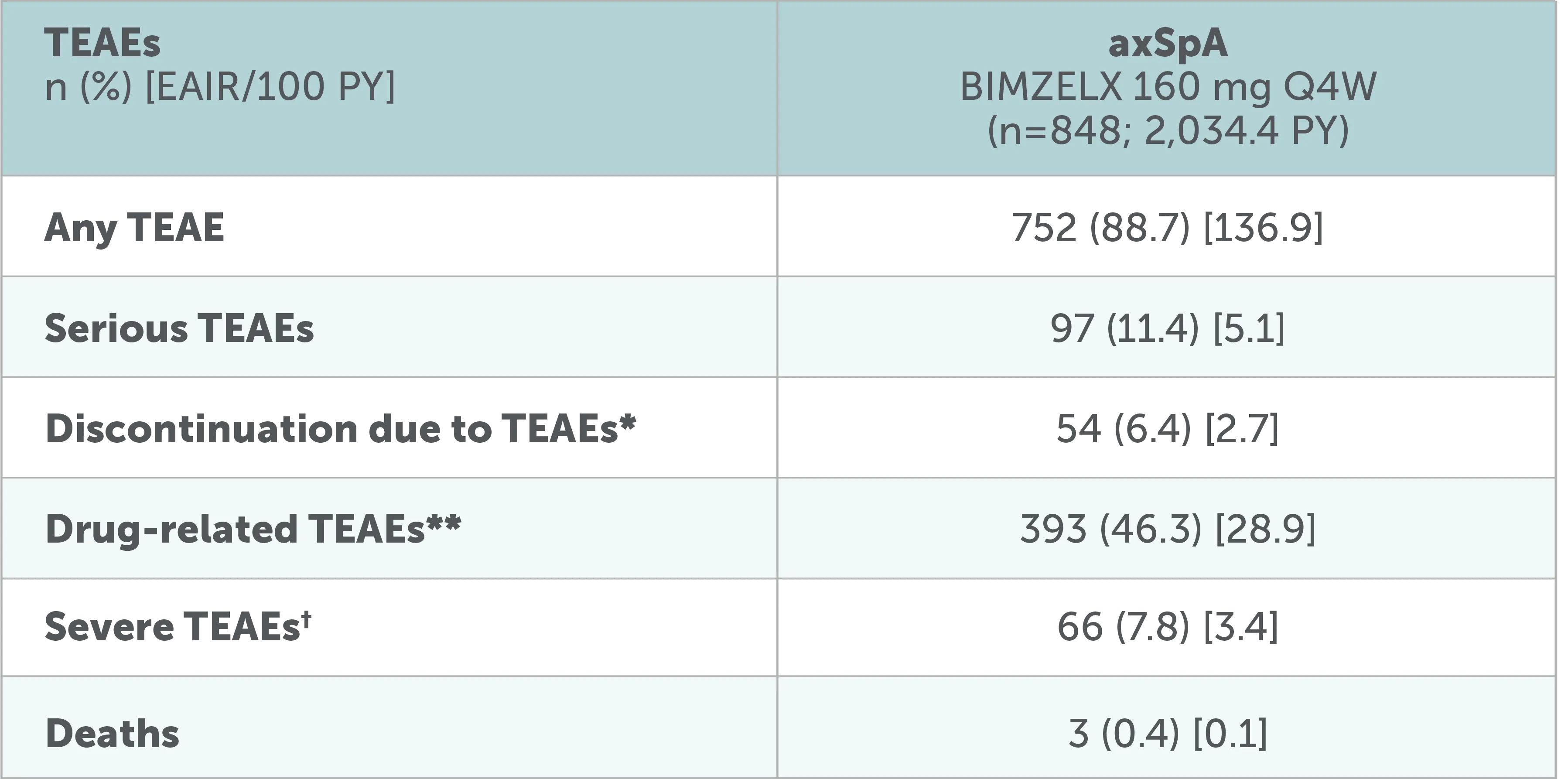
Adapted from Mease P, et al. RMD Open. 2025;11(2):e005026.
Data to the July 2022 data-cut shown, including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. After Week 16, all patients were aware that they were receiving active treatment, which may have affected the results. *Including TEAEs leading to death.26 **Per investigator assessment.26 †The intensity of TEAEs was assessed by the investigators as ‘mild’, ‘moderate’ or ‘severe’, independently from seriousness.
Pooled analysis of patients with axSpA (nr-axSpA or AS): Overview of AEs of special monitoring up to data-cut
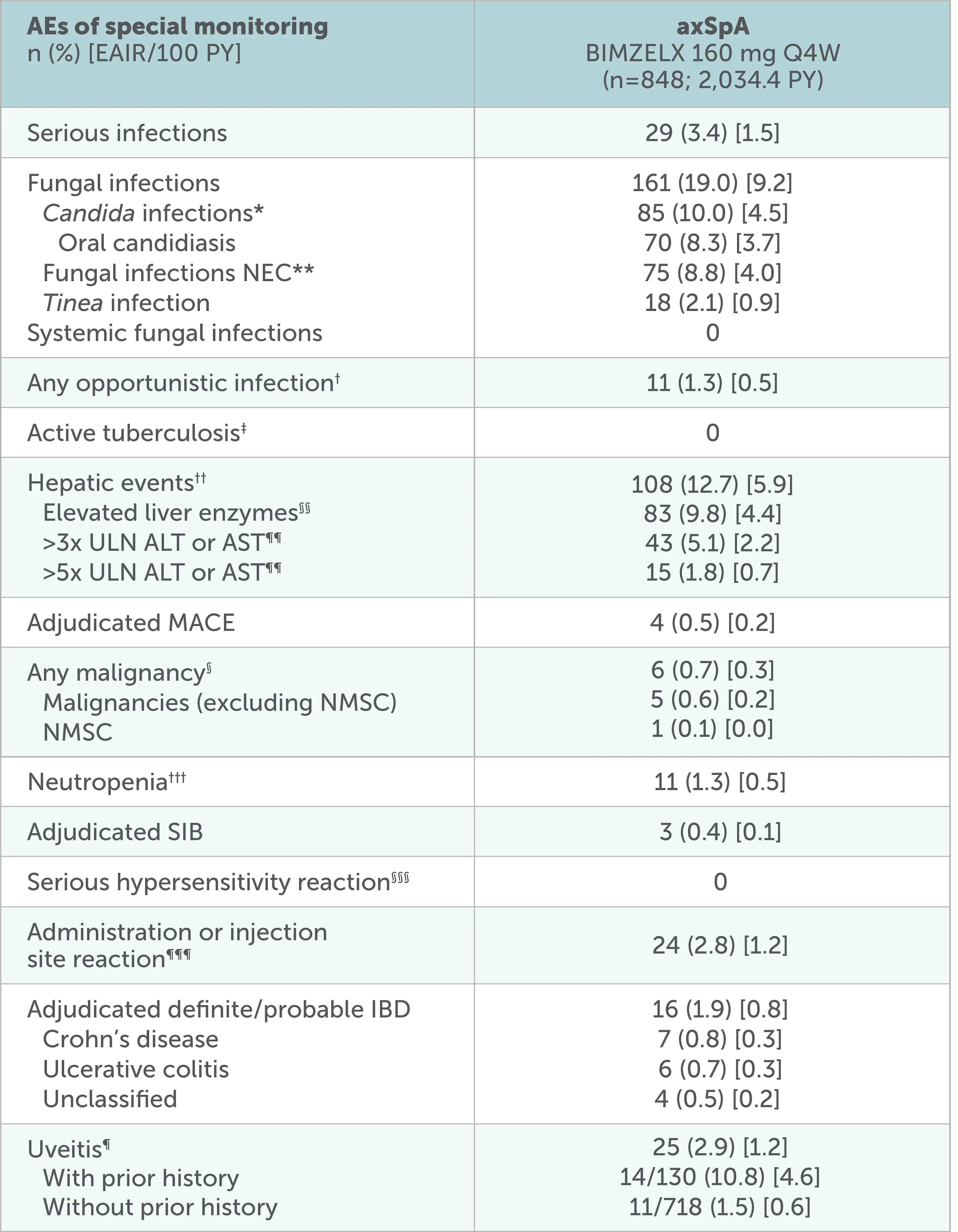
Adapted from Mease P, et al. RMD Open. 2025; 11(2):005026.
Data to the July 2022 data-cut shown, including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. After Week 16, all patients were aware that they were receiving active treatment, which may have affected the results.
*Shown by MedDRA preferred term in online supplemental table 2.26 **NEC denotes groupings of miscellaneous terms that do not readily fit into other hierarchical classifications within a specific SOC in the MedDRA.26 †Not including one serious case of oropharyngeal candidiasis in a patient with PsA, which was identified as an opportunistic infection after the July 2022 data-cut.26 ‡At baseline, 46 (5.4%) patients with axSpA and 34 (2.4%) patients with PsA had a history of ongoing/latent tuberculosis (phase 2B/3 pool).26 §Identified using the SMQ ‘Malignant tumours’.26 ¶Includes the preferred terms ‘Autoimmune uveitis’, ‘Iridocyclitis’, ‘Iritis’ and ‘Uveitis’.26 ††Includes events in the SMQ ‘Drug-related hepatic disorders—comprehensive search’, excluding the sub-SMQs ‘Liver neoplasms, benign (including cysts and polyps)’ and ‘Liver neoplasms, malignant and unspecified’.26 §§Elevated liver enzymes include the following preferred terms reported as adverse events: Increased/abnormal levels of ALT, AST, blood bilirubin, gamma-glutamyltransferase, hepatic enzyme, liver function test or transaminases.26 ¶¶n=411 for axSpA; n=1405 for PsA.26 †††Includes preferred terms identified based on UCB-defined search criteria.26 §§§Hypersensitivity reactions identified via the SMQ ‘Hypersensitivity’.26 ¶¶¶Identified using the HLTs ‘Administration site reactions NEC’ and ‘Injection site reactions’.26
No new safety signals were reported26
The three most common TEAEs were SARS-COV-2 (COVID-19) infection, nasopharyngitis, and upper respiratory tract infection
Incidence rate of oral candidiasis decreased over time and infrequently led to discontinuation
Low incidence of uveitis was seen in patients with axSpA across pooled phase 3 trials27
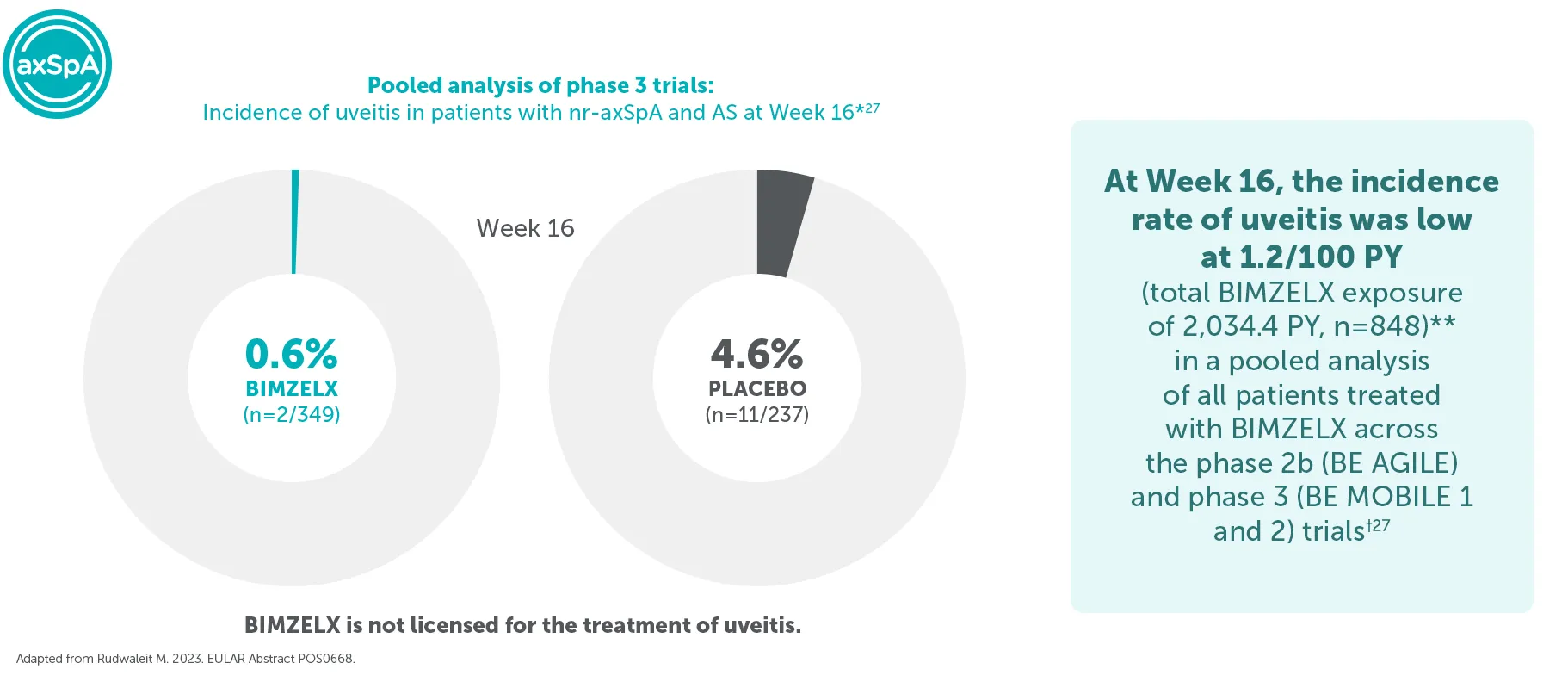
*Of 349 patients randomised to BIMZELX treatment across BE MOBILE 1 and BE MOBILE 2, 14.9% (n=52) had a history of uveitis at baseline (pooled data).6
**Of 848 patients randomised to BIMZELX across the phase 2b and phase 3 trials (pooled data), 15.3% (n=130) had a history of uveitis at baseline.27
†All uveitis events were mild-to-moderate and only one event led to discontinuation.27
Low incidence of uveitis was seen in patients with axSpA across pooled phase 2b/3 trials*28
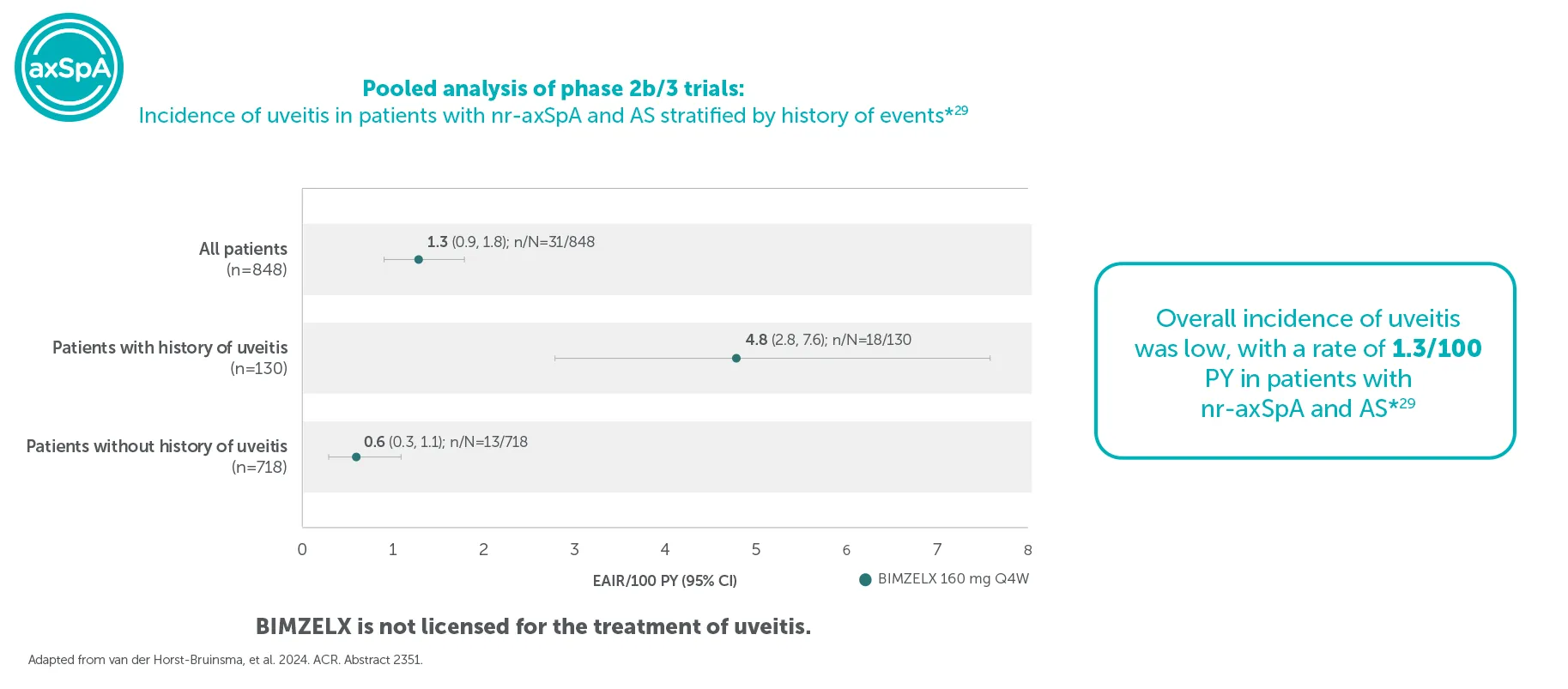
Pooled safety set including all patients who received ≥1 dose of BIMZELX 160 mg Q4W in the phase 2b/3 studies. Uveitis rates and EAIRs/100 PY were reported over median durations of approximately 2.8 years (axSpA) and 2.7 years (PsA); the data cut-off for both patient populations was set at July 2023.29
*In patients with nr-axSpA and AS across pooled data from phase 2b/3 trials, overall uveitis occurred in n=31/848 (3.7%) patients (EAIR [95% CI]: 1.3/100 PY [0.9, 1.8]).29 Overall exposure across the pooled phase 2b/3 trials was 2,514 PY.29 All events were mild or moderate and one led to treatment discontinuation.29
How to use
BIMZELX dosing regimen1

The recommended dose for adult patients with axSpA is 160 mg (given as one subcutaneous injection of 160 mg) every 4 weeks.7 The recommended dose for adult patients with active PsA is 160 mg (given as one subcutaneous injection of 160 mg) every 4 weeks.7 Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment.7
*The pre-filled syringe or pen may be stored at room temperature (up to 25 °C) for up to 25 days. Once removed from the refrigerator and stored under these conditions, discard after 25 days or by the expiry date printed on the container, whichever occurs first. A field for the date is provided on the carton to record the date removed from the refrigerator.7

IE-BK-2400125
Date of creation: October 2025