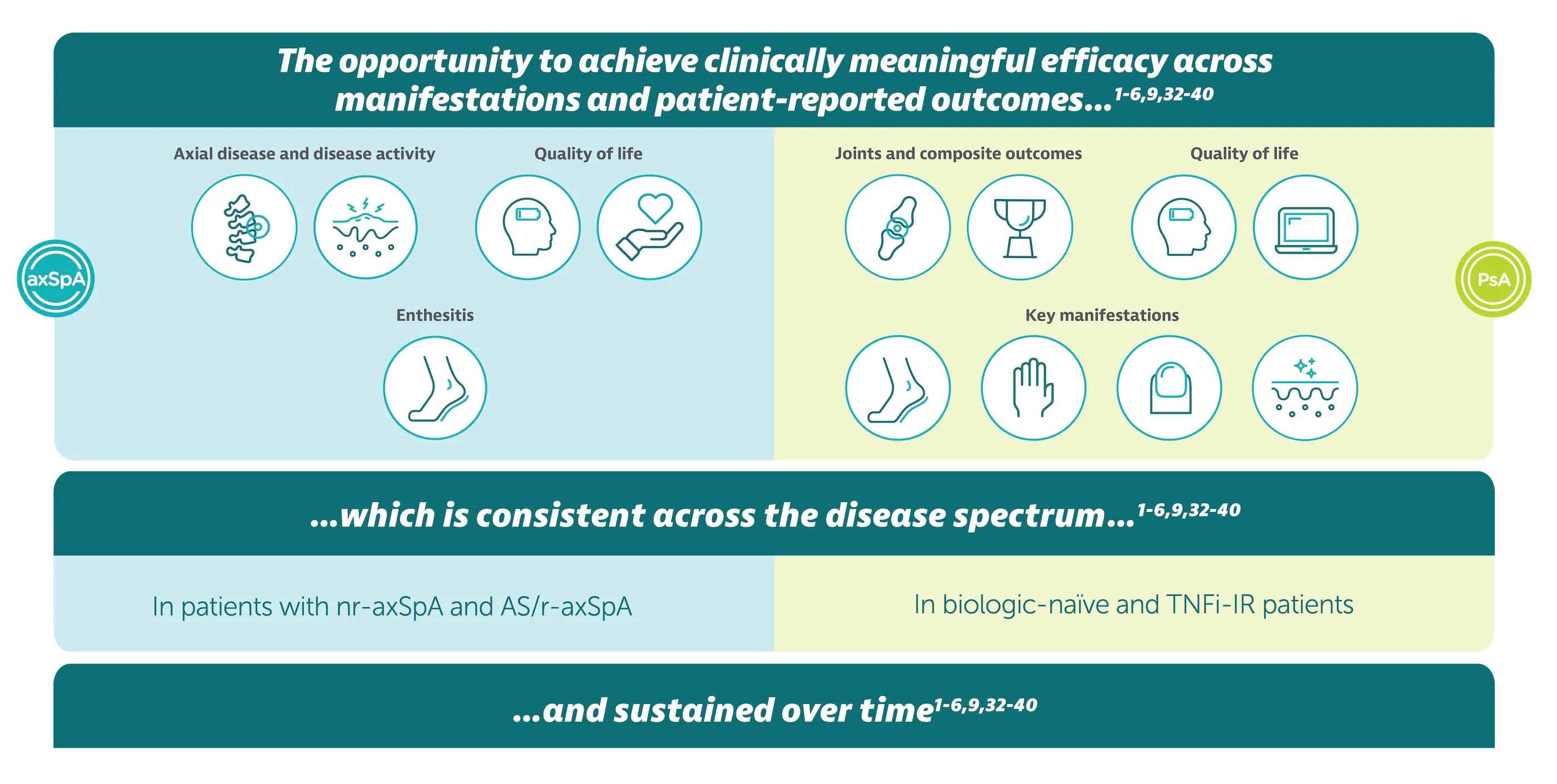
*Efficacy across manifestations means ACR 50 and PASI 90 for PsA, ASAS 40 and ASDAS <2.1 for axSpA. ACR 50 achieved by 43.9% (189/431) biologic-naïve and 43.4% (116/267) TNFi-IR patients with PsA at Week 16 (primary endpoint in BE OPTIMAL and BE COMPLETE; vs 10.0% (28/281) and 6.8% (9/133) with placebo, respectively; p<0.001),1–3 54.5% (235/431) and 51.7% (138/267) at Week 52, respectively (NRI analysis).4,5 ASAS 40 achieved by 47.7% (61/128) nr-axSpA patients and 44.8% (99/221) AS patients at Week 16, (primary endpoint in BE MOBILE 1/2; vs 21.4% (27/126) and 22.5% (25/111) with placebo, respectively; p<0.001);6 60.9% (78/128) and 58.4% (129/221) at Week 52, respectively (NRI analysis).3 Rapid onset demonstrated by numerically higher responder rates (ACR 50) observed vs placebo at Week 4 in BE OPTIMAL, 17.6% (76/431) vs 3.2% (9/281); and in BE COMPLETE, 16.1% (43/267) vs 1.5% (2/133), respectively (both nominal p<0.001).1–3 At Week 4, PASI 90 responses were numerically greater in BIMZELX-treated patients vs placebo in BE OPTIMAL, 19.8% (43/217) vs 4.3% (6/140), and in BE COMPLETE, 26.7% (47/176) vs 0% (0/88), respectively.1,2 At Week 52, PASI 90 achieved by 71.4% (155/217) and 74.4% (131/176) of BIMZELX-treated patients in BE OPTIMAL and BE COMPLETE, respectively (ranked secondary endpoint, NRI analysis; PASI response in patients with psoriasis involving at least 3% BSA at baseline).4,5 Rapid separation in ASAS 40 response rates observed within 1–2 weeks after a single dose of BIMZELX vs placebo; 16.4% (21/128) vs 1.6% (2/126) at Week 1 (nominal p<0.001) in BE MOBILE 1, and 16.7% (37/221) vs 7.2% (8/111) at Week 2 (nominal p=0.019) in BE MOBILE 2 (NRI analysis).3,7,8 At Week 2, ASDAS <2.1 achieved by 19.6% (25/128) of BIMZELX-treated patients vs 7.4% (n=9/126) placebo in BE MOBILE 1 and 24.6% (54/221) vs 8.4% (9/111) in BE MOBILE 2, respectively (MI).7,8 ASDAS <2.1 achieved by 61.6% (79/128) nr-axSpA patients and 57.1% (126/221) AS patients at Week 52 in BE MOBILE 1 and BE MOBILE 2 respectively (exploratory endpoint, MI analysis).3,9