
Bimzelx is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
The following data is for patients with Plaque Psoriasis
• 68.2% (n=238/349) achieved PASI 100 at Week 16 (placebo n=1/86)¥*1
• 75.9% (n=265/349) achieved PASI 75 at Week 4 (placebo n=1/86)¥* 1
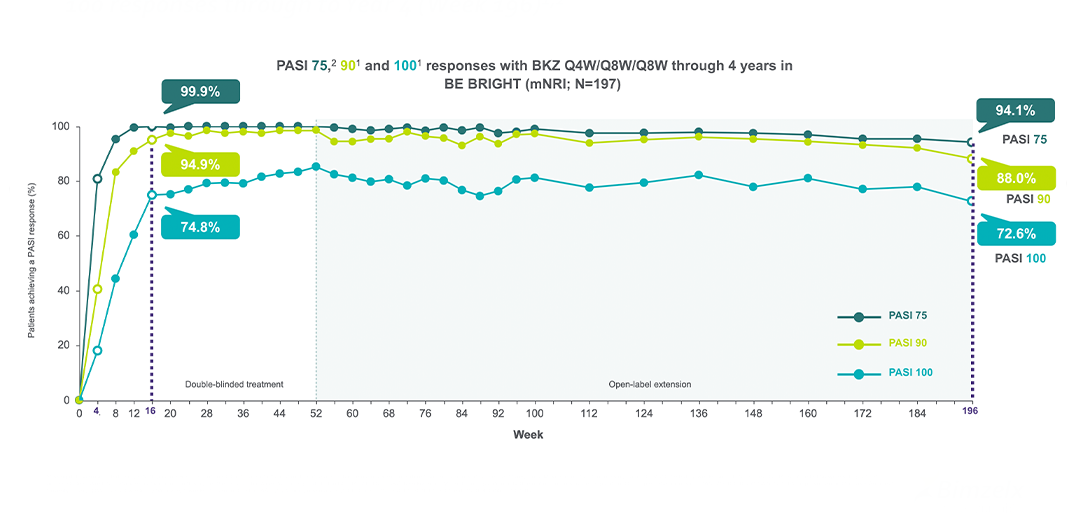
• 72.6% (N=197)§ achieved and maintained PASI 100 up to 4 years3
BIMZELX®▼ (bimekizumab) was well tolerated, the most frequently reported adverse reactions were: upper respiratory tract infections and oral candidiasis . Other common reported adverse reactions include tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, rash, dermatitis, eczema, acne, injection site reactions, fatigue, and vulvovaginal mycotic infection (including vulvovaginal candidiasis).2
Please refer to the SmPC for further information.
Footnotes:
¥co-primary endpoints PASI 90 and IGA 0/1 at Week 16 were met.
*secondary endpoints
§N= modified non-responder imputation (mNRI), missing data were imputed with mNRI (patients with missing data following treatment discontinuation due to lack of efficacy or a treatment-related adverse event were counted as non-responders; multiple imputation methodology was used for other missing data).
References:
1. Gordon et al. Lancet;2021;397;10273:475-486
2. BIMZELX® (bimekizumab). Summary of Product Characteristics.
3. Strober et al. AAD 2024; oral presentation.
Open Label Extension BE BRIGHT1
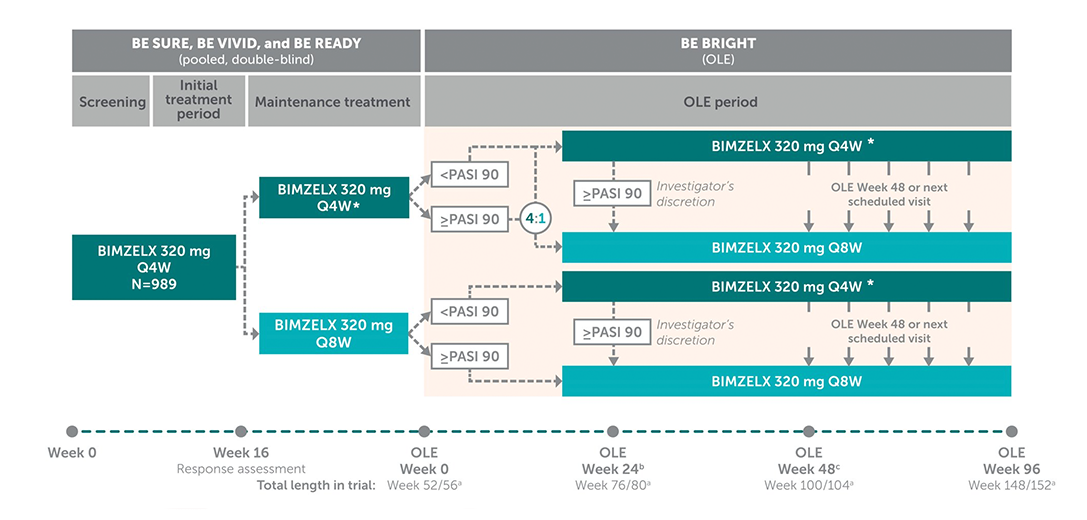
The BIMZELX clinical trials included some off-label treatment arms, the Q4W dosing after WEEK 16 does not reflect the licensed dosing regimen for patients <120kg.2 Please consult the SmPC for full details. The recommended dose for adult patients with plaque psoriasis is 320 mg at week 0, 4, 8, 12, 16 and every 8 weeks thereafter.2
Footnotes:
OLE, open-label extension; PASI 90/100, 90/100% improvement from baseline in Psoriasis Area and Severity Index; Q(2/4/8)W, every 2/4/8 weeks.
*Q4W dosing after Week 16 does not reflect the licensed dosing regimen for patients <120 kg. Please consult the SmPC for full details.2
a) BE SURE and BE READY had a duration of 56 weeks and BE VIVID had a duration of 52 weeks. b) At OLE Week 24, patients receiving BKZ who achieved PASI 90 could switch to receive BKZ Q8W at the discretion of the investigator. c) At OLE Week 48, or at the next scheduled clinical visit. All patients were re-assigned to BKZ Q8W, following protocol amendment.1 The recommended dose for adult patients with plaque psoriasis is 320 mg at Week 0, 4, 8, 12, 16 and every 8 weeks thereafter.2
References:
1. Strober, B., et al. [BE BRIGHT open label extension] Br J Dermatol. 2023; 188(6):749-59. 2. BIMZELX (bimekizumab). Summary of Product Characteristics.
BIMZELX demonstrated a complete and sustained response with PASI 75, 90 and 100 responses through to Year 4 (Week 196)1,2
Footnotes:
§N= modified non-responder imputation (mNRI), missing data were imputed with mNRI (patients with missing data following treatment discontinuation due to lack of efficacy or a treatment-related adverse event were counted as non-responders; multiple imputation methodology was used for other missing data).. BE VIVID lasted 52 weeks and BE SURE and BE READY lasted 56 weeks; to pool data across studies, Week 56 data were not included. In this figure, the period after Week 52 corresponds to the BE BRIGHT OLE. BKZ, bimekizumab; MI, multiple imputation; mNRI, modified non-responder imputation; OLE, open-label extension; PASI 75/90/100, ≥75%/≥90%/100% improvement in Psoriasis Area and Severity Index; Q4W. Every 4 weeks; Q8W, every 8 weeks.
References:
1. Strober B, et al. AAD 2024; oral presentation; 2. UCB DOF. PS0008, PS0009, PS0013 and PS0014 Table 6.19.3.1G.
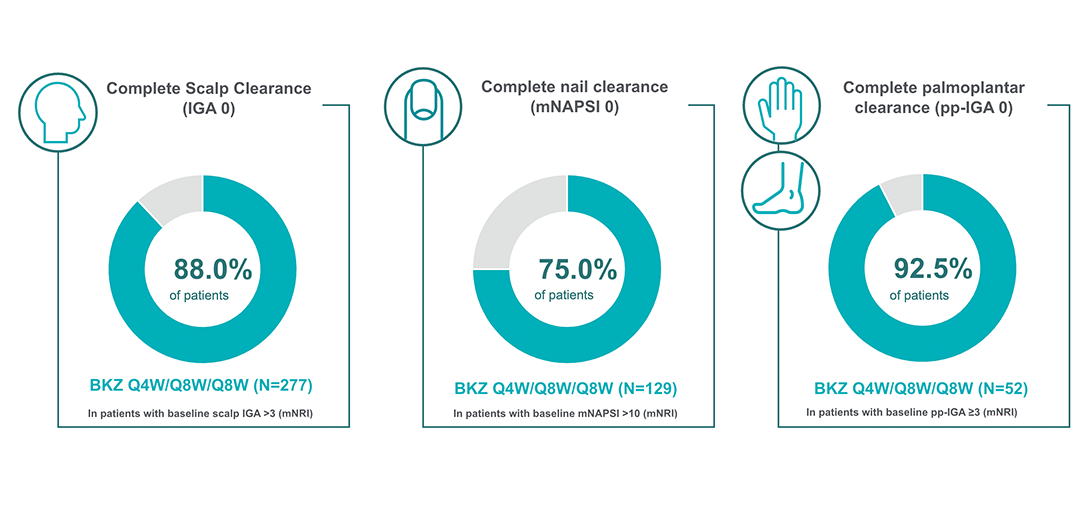
BIMZELX provides high rates of complete resolution across high-impact sites (scalp, nail, and palmoplantar psoriasis) through to Week 1441
Footnotes:
Post hoc analysis of data pooled from BE BRIGHT (96 weeks), and BE RADIANT (144 weeks) phase 3b trial. BKZ Q4W/Q8W/Q8W patients received BKZ 320mg Q4W to Week 16, then BKZ Q8W throughout the maintenance period and on OLE entry.1 Scalp IGA was a listed original secondary endpoint for BE READY and BE VIVID.2,3
BKZ, bimekizumab; mNAPSI, modified Nail Psoriasis Severity Index; mNRI, modified non-responder imputation; OLE, open-label extension; pp, palmoplantar psoriasis; Q4W, every 4 weeks; Q8W, every 8 weeks.
References:
1. Merola et al. EADV 2023; P2547; 2. Gordon et al. Lancet. 2021;397:475–86; 3. Reich et al. Lancet. 2021;397:487–98
IE-BK-2400177 | Date or preparation May 2024

IE-BK-2400177 | Date of preparation June 2024
